Published online Apr 28, 2008. doi: 10.3748/wjg.14.2494
Revised: December 11, 2007
Published online: April 28, 2008
AIM: To study the effect of 5-lipoxygenase/cyclooxy-genase-2 (5-LOX/COX-2) dual inhibitor 7-tert-butyl-2, 3-dihydro-3, 3-dimethyl substituted dihydrofuran 30 (DHDMBF30) on proliferation and apoptosis of the pancreatic cancer cell line Capan-2 and the effect of DHDMBF30 on human pancreatic cancer in a nude mouse model.
METHODS: Investigate the effect of 5-LOX/COX-2 dual inhibitor DHDMBF30 on proliferation and apoptosis of the pancreatic cancer cell line Capan-2 by RT-PCR, MTT assay, FCM and electron microscope. Cell line Capan-2 was inoculated percutaneously on the outer thigh of 12 nude mice. The VEGF mRNA of transplantation tumor was detected by RT-PCR.
RESULTS: DHDMBF30 inhibits the proliferation of cell line Capan2, reduces the expression of 5-LOX, COX-2 and VEGF. After Capan2 was treated with DHDMBF30, we found that the apoptosis peak of the experimental group was significantly higher than that of the contrast group (3.08 ± 1.89 vs 27.67 ± 0.52, P < 0.001). The tumor weight of the DHDMBF30 group was significantly lower than PBS control groups (1.35 ± 0.47 vs 2.92 ± 0.73, P < 0.01). Expression of VEGF in the DHDMBF30 group was significantly decreased.
CONCLUSION: DHDMBF30 inhibits the proliferation of the pancreatic cell line Capan2, and induces apoptosis and inhibits the growth of pancreatic cancer in nude mice.
- Citation: Zhang B, Wang CL, Zhao WH, Lv M, Wang CY, Zhong WX, Zhou WY, Yu WS, Zhang Y, Li S. Effect of 5-LOX/COX-2 common inhibitor DHDMBF30 on pancreatic cancer cell Capan2. World J Gastroenterol 2008; 14(16): 2494-2500
- URL: https://www.wjgnet.com/1007-9327/full/v14/i16/2494.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2494
Through many years of practice and research, the treatment of pancreatic cancer has made some advancement in the areas of surgical operation, radiotherapy and chemotherapy, but the therapeutic effect is not yet satisfied enough, especially in the patients of advanced stages, and the remission rate or survival rate has not been improved significantly. At present, the major treatment method is the combined therapy, such as surgical operation combined with chemotherapy and (/or) radiotherapy etc. The prevention and treatment mechanism of non-steroid anti-inflammatory drug (NSAIDs) against tumors are still not clear now, but it is certain that NSAIDs can inhibit cyclooxygenase-2 (COX-2), which is the key enzyme in the synthesis of prostaglandin (PG). Some experiments demonstrated that the selective COX-2 inhibitors could inhibit the tumor cell proliferation, induce apoptosis and inhibit the generation of new vessels for tumors[12]. In recent years, a number of research studies have demonstrated that NSAIDs could inhibit 5-lipoxygenase (5-LOX), thereby inhibiting proliferation and inducing apoptosis of many malignant tumors[34]. In our early phase study, it was demonstrated that the expression rates of 5-LOX and COX-2 in the pancreatic cancer tissues were respectively 74.3% and 80%[56], and all the pancreatic cancer tissues expressed 5-LOX or COX-2, sometimes expressed both, which supported that there were crossed and complementary expressions, so the designed 5-LOX/COX-2 dual inhibitor can exert a synergistic effect. So we chose the 5-LOX/COX-2 dual inhibitor DHDMBF30 to act against pancreatic cancer cells, and observed the anticancer effects in order to provide a foundation for its clinical application.
The human pancreatic cancer cell Capan-2 was obtained from the Pathology Teaching Research Department of Peking Union Medical College Hospital; pancreatic cancer cell medium RPMI1640 (GIBCO company) contained 20 mmol/L NaHCO3, 100 U/mL penicillin, 100 U/mL streptomycin, 10% calf serum (Hangzhou Sijiqing Biological Engineering Materials Co, Ltd); 0.25% trypsin (Sigma company). The primers were synthesized by the Shanghai Institute of Biochemistry, Chinese Academy of Sciences, RNA extract reagent TrizolR and RT-PCR kits were bought from GIBCO company. DHDMBF30 was bought from Procter & Gamble Pharmaceuticals (Ohio). Experimental animals: BALB/C nu hairless mice were bought from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences. The mice were 4-6 wk old, and there were 6 males and 6 females.
Pancreatic cancer cell strain Capan-2 was cultured in RPMI1640 medium containing 20% calf serum, and the cell strain was generally cultured in 37°C incubator with 5% CO2. Digestion of the strains was performed with 0.25% trypsin and serially sub-cultivated after 70%-80% of them coalesced during the adherent growth process. Next, we inoculated Capan-2 cells into the 100 mL culture flask at a dose of 106/flask after the strains were digested, then divided the strains into control group and experimental group, cultured for 3 d, and changed the medium when the cells entered into the exponential division phase, then added 12 &mgr;mol/L DHDMBF30 into the culture flask in the experimental group and cultured for another 24 h.
Six mice in each group were put into a rearing cage. Experimental group: on the second day after cell inoculation each mouse was subcutaneously injected with 0.2 mL (12 &mgr;mol/L) DHDMBF30 in the inoculation area of tumor cells twice a week for 3 wk; PBS control group: the injection time, site and volume were the same as the treatment group. After the tumor could be touched, we measured the long and short diameters of the tumor every 3 d. Hairless mice were sacrificed 35 d later, the tumor mass measured and tissue fixed with 10% formalin. Immunohistochemistry and pathological examinations were then performed.
Cells were cultured in 1640 liquid culture media containing 20% calf serum, and transferred into 1640 liquid without calf serum after 24 h, prepared at a 5 × 104/mL cell suspension and added into a 96 well plate with 0.1 mL suspension in each well. For every dose group, a set of 3 auxiliary wells were prepared and we added DHDMBF30 of different concentrations, set the final concentrations at 5 &mgr;mol/L, 10 &mgr;mol/L, 15 &mgr;mol/L, and 20 &mgr;mol/L with the total volume of 100 &mgr;L and continued to culture the cells. Added 20 &mgr;L 5 mg/mL MTT 4 h before stopping drug at 12, 24, 36 and 48 h, then returned the culture plate into the CO2 incubator at 37°C for 4 h (the cells would deoxidize the yellow MTT to hyacinthine crystallization under the action of SDH). Next we aspirated and discarded 90% of the culture liquid in the wells, added 100 &mgr;L DMSO into each well and thoroughly shook the wells for 10 min to completely dissolve the crystals. We determined the A values of each well at the wavelength of 570 nm, and calculated the GIR of the cells. We repeated the experiment 6 times. Calculated GIR with the formula:
GIR (%) = A value of control pore-A value of experimental pore × 100% A value of the control pore.
After the cells were cultured in the 1640 liquid culture media containing 20% calf serum for 24 h, the liquid was replaced with the 1640 liquid culture media without calf serum for another 24 h culture, and finally the cells were digested with 0.25% trypsin and 0.02% EDTA to prepare a unicell suspension with the cell concentration at about 5 × 105/mL. The suspension was plated into a 6-well plate with 1 mL suspension in each well. The control group was drug free; 12 &mgr;mol/L DHDMBF30 was added to the experimental group and cultured for 24 h again. We repeated this experiment 3 times. Suspensions were also prepared and stained with propidium iodide (PI), then injected the suspension.
Cells from the control and experimental groups were fixed, dehydrated, replaced, soaked and embedded, prepared semi thin sections, stained with electrons and observed for ultra structural structures.
Process: (1) Collect the cells from the control and experimental groups: take cells cultured for 24 h, digest with 0.25% trypsin to prepare unicell suspension, centrifuge at 500 r/min for 10 min, collect the cell precipitates and rinse with 0.01mmol/L PBS (pH = 7.4) for one time, then store at -70°C; (2) Extraction of total RNA of the cell: extract the total RNA with the TrizolR kit, learn about the integrity of the extracted RNA by formaldehyde denaturing gel electrophoresis, determine A260 nm and A280 nm values by ultraviolet spectrophotometer to analyze its purity, and fix quantity at the same time; (3) The synthesis of cDNA: mix 1 &mgr;g RNA sample with 1.2 &mgr;L random hexamer primer, put them into an iced bath after annealing at 70°C for 5 min, then add 4 &mgr;L reverse transcriptase buffer, 1 &mgr;L 20-40 Mu/L Rnasin and 2 &mgr;L 10 mmol/L dNTP, mix them completely at 25°C for 5 min; add 2 &mgr;L MmuLV reverse transcriptase finally and add water to 20 &mgr;L volume, heat at 25°C for 5 min, 37°C for 60 min and 70°C for 10 min, stop the reaction and store the reagents at 4°C; (4) PCR: the primer sequence of 5-LOX was 5’-CCCGGGGCATGGAGAGCA-3’, 5’-GCGGTCGGGCAGCGTGTC-3’; COX-2: 5’-TTCAAATGAGATTGTGGGAAAATTGCT-3’, 5’-AGATCATCTCTGCCTGAGTATCTT-3’; VEGF: 5’-TTGCTGCTCTACCTCCAC-3’, 5’-AATGCTTTCTCCGCTCTG-3’; β-actin: 5’-GTGGGGCGCCCCAGGCACCA-3’, 5’-CTCCTTAATGTCACGCACGATTT-3’. The lengths of the amplification fragments were 416, 305, 418, 500 bp respectively; the reaction systems were 0.8 &mgr;L cDNA, 2 &mgr;L 10 × Taq enzyme buffer, 0.4 &mgr;L 10 mol/L dNTP, 0.8 &mgr;L 25 mmol/L MgCl2, the up and down stream primers were 0.4 &mgr;L and 0.5 &mgr;L Taq enzymes, and the water was added to 20 &mgr;L. PCR conditions: denature at 95°C for 45 s, anneal at 54°C for 90 s, elongate at 72°C for 90 s, and repeat for 30 cycles. At last, elongate at 54°C for 2 min and 72°C for 3 min. After the reaction, take out 10 &mgr;L amplification products to 17 g/L agarose gel for electrophoretic analysis. In order to compare the intensities of expression levels, scan the amplification bands by image analyzer for semi-quantitative analysis.
We detected the VEGF mRNA of the transplanted tumor by RT-PCR, and the VEGF protein by the immunohistochemistry SP method. We measured the absorbance of the immunohistochemistry stained slide by microspectrophotometer, chose 100 cells randomly in each slide, scanned the absorbance value of each cell at the wavelength of 460 nm and calculated the expression area, worked out their means to express the relative content of VEGF protein.
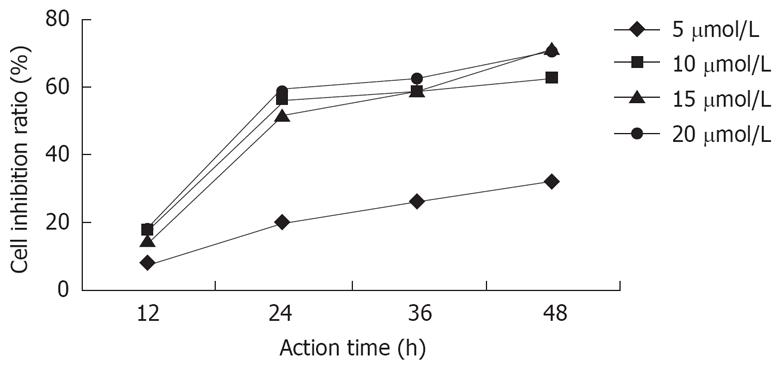
After the actions of DHDMBF30 of different concentrations against Capan-2 cells for 12 h, 24 h, 36 h, 48 h, with the concentration increasing and the duration prolonging, the inhibition was strengthened, but after the concentration and the prolonged duration reached certain values, the inhibition ratio didn’t increase anymore, but a plateau appeared, the IC50 was 12 &mgr;mol/L and the inhibition ratio didn’t increase after 24 h (Figure 1).
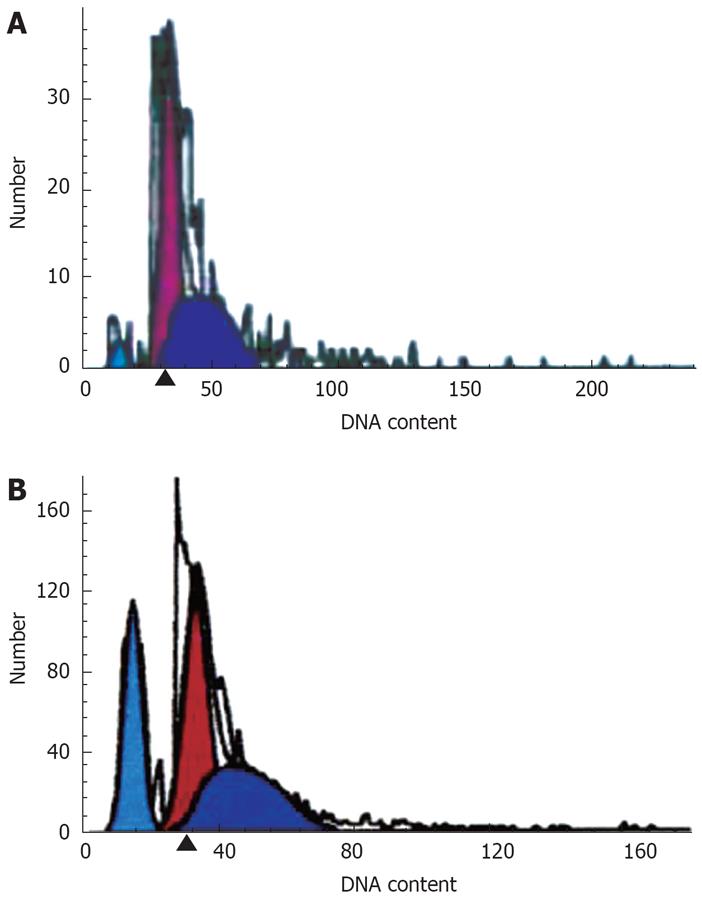
There was an apoptosis peak of Capan-2 cells (3.08 ± 1.89) before G0/G1, and the peak (27.67 ± 0.52) was higher after the treatment by DHDMBF30, which was significantly different compared with the control group (P < 0.001) (Figure 2).
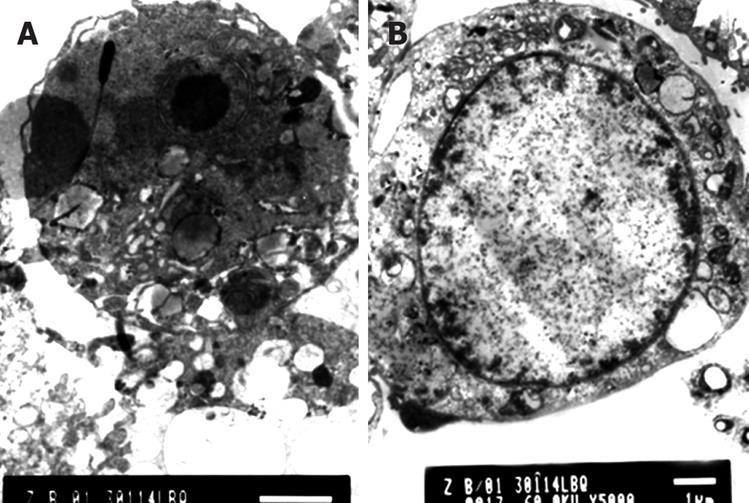
The Golgi's complexes in the Capan-2 cells of the control group were developed, there were a lot of rough endoplasmic reticula, chondrosomes were swelling, the karyoplasmic ratio was high, the karyotheca showed depressed wrinkles, and the chromatins were abundant in the nucleus and nucleoli were large. After a 12 h treatment with DHDMBF30, microvilli of most of the cell surface were reduced, the cell volume was decreased and the cytoplasm concentrated, a large number of vacuolar degenerations appeared in some cells; the cell nucleus was shrunk, the karyotheca existed but the nucleolus disappeared, the dyeing of chromatins was darkened and the latter congregated into masses adjoining the karyotheca; 24 h later, the membrane became smooth and the cytoplasm continued to condense, there were concentrated nuclear fragments, and some cells produced apoptotic bodies through budding. The following figures show the nuclear fragments enclosed by membrane structures and degenerated cell organs (Figure 3).
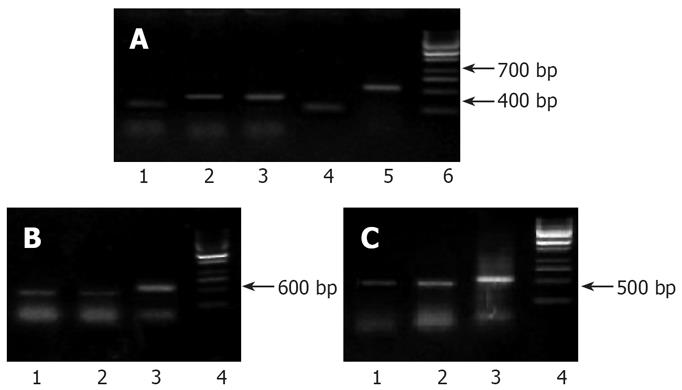
In the electrophoretic analysis on RT-PCR products of pancreatic cancer cell strains in the control group, one 416 bp and one 304 bp amplification bands could be observed; the 416 bp and 304 bp amplification bands also existed in the experimental group, but the brightness was lowered (Figure 4A), which demonstrated that there were expressions of 5-LOX and COX-2 genes in the Capan-2 cells of pancreatic cancer, and the inhibitor decreased their expressions.
A 418 bp band was obtained in the electrophoretic analysis of RT-PCR products from the pancreatic cancer cell strains in the control group, and there was also a specific 418 bp amplification band with a lower brightness in the experimental group (Figure 4B).
The growth conditions of the transplant tumor: The subcutaneous tumor began to form 2-3 wk after the inoculation, and the volume reached 2-3 cm 4-5 wk later. In the PBS control group, the transplant tumor formed in all the inoculation regions, and all of the tumor volumes were large, the tumor formulation rate was 100% (6/6); while the formulation rate in the experimental group was 67% (4/6) and the volume was small. The subcutaneous tumors grew slowly and the tumor bodies were hard, they were all fixed on the lateral side of the thigh. When the diameter of the tumor body reached 25 mm in the advanced stage, gross nutrition vessels could be observed on the skin upon the tumor body.
The growth of transplant tumor under the skin of the hairless mice and the change of tumor weight: the growth rate of the transplant pancreatic tumor in hairless mice of the experimental group was obviously lower than the PBS control group; the tumors could be touched on the 14th d in the PBS group, while it was the 20th d in the experimental group. The average weight of the hairless mice in the experimental group was (1.50 ± 0.52) g, which was significantly lower than (2.18 ± 0.96) g of the PBS control group (P < 0.01) (Table 1).
The detection results of VEGF mRNA: The 418 bp band amplified in transplant tumor indicated the expression of the VEGF mRNA, and the brightness of VEGF mRNA band in the DHDMBF30 treating group was obviously lowered (Figure 4C).
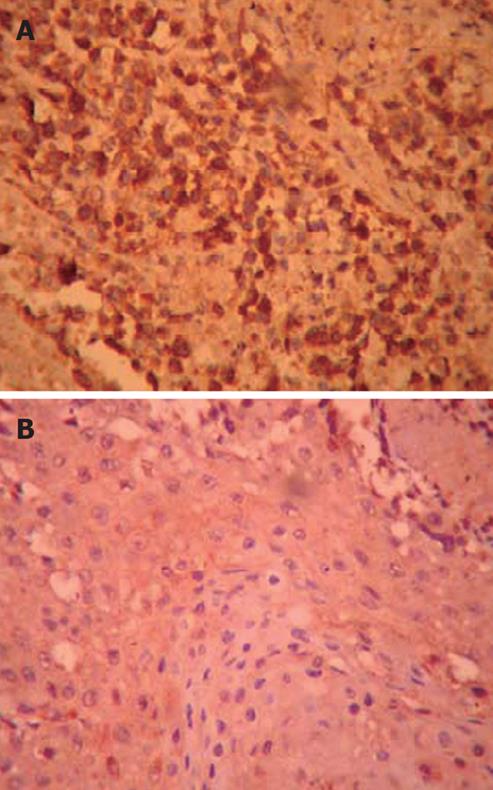
The expression of VEGF protein: The results of immunohistochemistry showed that the VEGF expression was positive in the transplant human pancreatic tumor in the hairless mice of the control group. Positive buffy granules could be seen in the cytoplasm of the pancreatic cancer cells; the cytoplasm of pancreatic cancer cells of the DHDMBF30 group stained lightly, and the stained cell number was small, so it was weakly positive. Please refer to the table for the comparison of absorbance and expression area (Figure 5; Table 2).
NSAIDs mainly act against the mechanism of arachidonic acid (AA) to produce a marked effect. There are two major metabolic enzymes of AA: lipoxygenase (LOXs) and cyclooxygenase (COXs)[78]. It has been found in a large number of studies in recent years that, 5-LOX and COX-2 of the lipoxygenase and cyclooxygenase can not only act against infections, but also relate to the pathogenesis, development and transfer of the tumors[9–14]. Luo et al[15] reported that 5-LOX in the endothelial cells could promote the generation of cancer, colonitis, psoriasis, etc. 0hno, et al[16–19] determined the expression quantity of COX-1 and COX-2 of the surgical sample obtained from gastric cancer in 33 patients by RT-PCR, stained the COX-2 antibody in the sample with immunohistochemistry staining and did routine histology examination at the same time, and they found that the COX-2 expression index in the gastric cancer was significantly higher than the normal mucosa (3.4-0.7 vs 2.2-0.7, P < 0.05), and the index of COX-2 increased with the infiltration of gastric cancer advancing.
Tenidap developed by Pfizer was a drug with a double inhibition effect against COX/5-LOX, and it was demonstrated in the clinical trials that its therapeutic effect was superior over other NSAIDs, such as diclofenac sodium, Naproxen, etc, but its hepatotoxicity was high, so it was withdrawn before long it came into the market. There was still a study in which the effect of the combination of COX-2 and 5-LOX inhibitors against the tumor was observed. At present, the researchers dedicated themselves to research and develop the selective 5-LOX/COX-2 double inhibitors, the DHDMBF had a certain double inhibition effect[20–22], through modifying the cycles or sites, researchers obtained more than 30 chemicals, among which the effect of DHDMBF30 was good both in vivo and in vitro and its inhibition ratio against COX-1 was extremely low, but there was no study on its effect against tumors. We found in our study that, 24 h after DHDMBF30 of different concentrations acted against Capan-2 cells, the inhibition ratio of the higher concentration group to Capan-2 was obviously increased; after the DHDMBF30 treatment on Capan-2 cells, the cellular morphologies were obviously altered, the granules and vacuoles were increased, some cells contracted and became rounder, the cell membrane shrunk, many cells dropped from the glass wall and were suspended in the culture liquid, and the dropped cells gradually increased with duration in culture and increasing drug concentration. Twenty-four hours later, the cellular membrane became smoother and the cytoplasm continued to condense, concentrated nuclear fragments could be observed and apoptotic bodies were produced in some cells through budding. In the detection of Capan-2 cell apoptosis by flow cytometry, it was found that there was an apoptosis peak of the Capan-2 cells in the control group before G0/G1, and the peak was heightened after the treatment of DHDMBF30, the results were significantly different compared with the control group[2324].
In former studies, COX-2 or 5-LOX inhibitors were separately applied to interfere in the tumor cells, and their effects were observed. Recently, some researchers have combined selective COX-2, 5-LOX inhibitors or broad-spectrum inhibitors to act against the tumor cells to maximally kill the tumor cells, but with increasing dose, the side effects were certainly increased. Thereby, we applied one drug to observe its effect in order to obtain the optimal therapeutic effect. As a common clinically applied and symptomatic treating drug for arthritis, the side effects of NSAIDs in the gastrointestinal tract were common, so the pharmaceutical chemists at home and abroad had always tried to find a new anti-inflammatory agent of high efficiency and low toxicity. The inhibiting concentration ratio (IC50) of NSAIDs against COX-2/COX-1 could reflect the side effects of the drug for the applied anti-inflammatory dose: if the COX-2/COX-1 IC50 was less than 1, the drug could selectively inhibit COX-2, the anti-inflammatory effect was strong while the side effects on stomach and kidney were less; if the COX-2/COX-1 IC50 was more than 1, the drug could strongly inhibit COX-1 with more side effects. Garcia Rodrigues and Jick[25] publicized epidemiological statistics data of the side effects of NSAIDs, the results confirmed that the inhibition concentration ratio of COX-2/COX-1 and side effects of gastrointestinal tract had a parallel relationship. The selective COX-2 inhibitor meloxicam, which had been registered in our country and some other countries, was designed to treat rheumatoid arthritis and ostarthritis, it had stronger anti-inflammatory effects and fewer side effects, and its inhibiting concentration ratio to COX-2/COX-1 was 0.07. The DHDMBF30, which we chose, was synthesized in recent years, its inhibiting concentration ratio to COX-2/COX-1 was 0.03, and the effect was good in both in vivo and in vitro experiments[26–30].
By using RT-PCR, we confirmed Capan2 cell expressed 5-LOX mRNA, COX-2 mRNA and VEGF mRNA, and the expression of 5-LOX mRNA, COX-2 mRNA2, VEGF mRNA was obviously decreased after the treatment of DHDMBF30; morphologic changes of shrinkage and apoptosis were also observed by microscope and electron microscopy; flow cytometry detected that the apoptosis rate of Capan2 cells was significantly increased. DHDMBF30 could inhibit the proliferation of Capan2 cells and induce its apoptosis, down regulate the vascular endothelial growth factors; its effect was better than the single application of COX-2 or 5-LOX inhibitor, and it could make up the ineffective result if the 5-LOX or COX-2 was not expressed.
The tumor formation rate, the weight and volume of the tumor body of the hairless mice in the DHDMBF30 treating group were all significantly less than those of the PBS control group, and no side effects were observed at the same time. The VEGF expression of the transplant pancreatic tumors in hairless mice was decreased, and the division, growth and assorting effects on the vascular endothelial cells in the tissues were lowered. Meanwhile, it inhibited the increase of vasopermeability and decreased the exudation of matrix ingredients such as blood plasma from the tissue, the foundations on which the vascular endothelial cells and tumor cells relied were eliminated, and thereby the goal of tumor growth inhibition was achieved.
The regional injection of DHDMBF30 could inhibit the growth of pancreatic cancer, and the manifestations were the prolonged incubating stages of the tumor and the lighter tumor weight (P < 0.01). But DHDMBF30 still could not completely inhibit the tumor growth, which might be related to the dose, and it was demonstrated that the dose-effect relationship and time-effect relationship should be further studied.
The prevention and treatment mechanism of non-steroid anti-inflammatory drug (NSAIDs) against tumors are still not clear now. In our early phase study, it was demonstrated that the expression rates of 5-lipoxygenase (5-LOX) and cyclooxygenase-2 (COX-2) in the pancreatic cancer tissues were respectively 74.3% and 80%[56], and all the pancreatic cancer tissues expressed 5-LOX or COX-2, sometimes expressed both, which supported that there were crossed and complementary expressions, so the designed 5-LOX/COX-2 dual inhibitor can exert a synergic effect.
It has been found in a large number of studies in the recent years that, 5-LOX and COX-2 of the lipoxygenase and cyclooxygenase can not only act against infections, but also relate with the pathogenesis, development and transfer of the tumors[9–14]. At present, the researchers dedicated themselves to research and develop the selective 5-LOX/COX-2 double inhibitors, the 7-tert-butyl-2,3-dihydro-3,3-dimethyl substituted dihydrofuran (DHDMBF) had certain double inhibition effect[20–22], through modifying the cycles or sites, researchers obtained more than 30 chemicals, among which the effect of DHDMBF30 was good both in vivo and in vitro and its inhibition ratio against COX-1 was extremely low, but there was no study on its effect against tumors.
DHDMBF30 inhibits the proliferation of Capan2 cells and induces its apoptosis, down regulates the vascular endothelial growth factors; its effect was better than the single application of COX-2 or 5-LOX inhibitor, and it could make up the ineffective result if the 5-LOX or COX-2 was not expressed.
The regional injection of DHDMBF30 could inhibit the growth of pancreatic cancer, and the manifestations were the prolonged incubating stages of the tumor and the lighter tumor weight (P < 0.01). We chose the 5-LOX/COX-2 dual inhibitor DHDMBF30 to act against pancreatic cancer ,and observed its anticancer effects.
NSAIDs: Non-steroid anti-inflammatory drug. DHDMBF: The selective 5-LOX/COX-2 double inhibitors, the 7-tert-butyl-2, 3-dihydro-3, 3-dimethyl substituted dihydrofuran.
This article is interesting which studied the effect of a kind of 5-LOX/COX-2 dual inhibitor on proliferation and apoptosis of pancreatic cancer cell line Capan2 and the effect of it on human pancreatic cancer in nude mice model. It provided a foundation for its clinical application.
| 1. | Bommareddy A, Arasada BL, Mathees DP, Dwivedi C. Chemopreventive effects of dietary flaxseed on colon tumor development. Nutr Cancer. 2006;54:216-222. [Cited in This Article: ] |
| 2. | Yoshikawa R, Fujiwara Y, Koishi K, Kojima S, Matsumoto T, Yanagi H, Yamamura T, Hashimoto-Tamaoki T, Nishigami T, Tsujimura T. Cyclooxygenase-2 expression after preoperative chemoradiotherapy correlates with more frequent esophageal cancer recurrence. World J Gastroenterol. 2007;13:2283-2288. [Cited in This Article: ] |
| 3. | Ferrera P, Arias C. Differential effects of COX inhibitors against beta-amyloid-induced neurotoxicity in human neuroblastoma cells. Neurochem Int. 2005;47:589-596. [Cited in This Article: ] |
| 4. | Cianchi F, Cortesini C, Magnelli L, Fanti E, Papucci L, Schiavone N, Messerini L, Vannacci A, Capaccioli S, Perna F. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol Cancer Ther. 2006;5:2716-2726. [Cited in This Article: ] |
| 5. | Zhang B, Shi XT, Yi LH, Li ZY, Yu JM. Expression of 5-lipoxygenase in pancreatic cancer and its clinical significance. Zhonghua Zhongliu Fangzhi Zazhi. 2004;11:68-70. [Cited in This Article: ] |
| 6. | Gregor JI, Kilian M, Heukamp I, Kiewert C, Kristiansen G, Schimke I, Walz MK, Jacobi CA, Wenger FA. Effects of selective COX-2 and 5-LOX inhibition on prostaglandin and leukotriene synthesis in ductal pancreatic cancer in Syrian hamster. Prostaglandins Leukot Essent Fatty Acids. 2005;73:89-97. [Cited in This Article: ] |
| 7. | Schroeder CP, Yang P, Newman RA, Lotan R. Simultaneous inhibition of COX-2 and 5-LOX activities augments growth arrest and death of premalignant and malignant human lung cell lines. J Exp Ther Oncol. 2007;6:183-192. [Cited in This Article: ] |
| 8. | Yang K, Ma W, Liang H, Ouyang Q, Tang C, Lai L. Dynamic simulations on the arachidonic acid metabolic network. PLoS Comput Biol. 2007;3:e55. [Cited in This Article: ] |
| 9. | Sun Z, Sood S, Li N, Ramji D, Yang P, Newman RA, Yang CS, Chen X. Involvement of the 5-lipoxygenase/leukotriene A4 hydrolase pathway in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamster cheek pouch, and inhibition of carcinogenesis by its inhibitors. Carcinogenesis. 2006;27:1902-1908. [Cited in This Article: ] |
| 10. | Luo M, Lee S, Brock TG. Leukotriene synthesis by epithelial cells. Histol Histopathol. 2003;18:587-595. [Cited in This Article: ] |
| 11. | Vidal C, Gomez-Hernandez A, Sanchez-Galan E, Gonzalez A, Ortega L, Gomez-Gerique JA, Tunon J, Egido J. Licofelone, a balanced inhibitor of cyclooxygenase and 5-lipoxygenase, reduces inflammation in a rabbit model of atherosclerosis. J Pharmacol Exp Ther. 2007;320:108-116. [Cited in This Article: ] |
| 12. | Zhi H, Zhang J, Hu G, Lu J, Wang X, Zhou C, Wu M, Liu Z. The deregulation of arachidonic acid metabolism-related genes in human esophageal squamous cell carcinoma. Int J Cancer. 2003;106:327-3233. [Cited in This Article: ] |
| 13. | de Gaetano G, Donati MB, Cerletti C. Prevention of thrombosis and vascular inflammation: benefits and limitations of selective or combined COX-1, COX-2 and 5-LOX inhibitors. Trends Pharmacol Sci. 2003;24:245-252. [Cited in This Article: ] |
| 14. | Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501-509. [Cited in This Article: ] |
| 15. | Park S, Han SU, Lee KM, Park KH, Cho SW, Hahm KB. 5-LOX inhibitor modulates the inflammatory responses provoked by Helicobacter pylori infection. Helicobacter. 2007;12:49-58. [Cited in This Article: ] |
| 16. | Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876-1881. [Cited in This Article: ] |
| 17. | Yang YY, Lin HC, Huang YT, Lee TY, Hou MC, Wang YW, Lee FY, Lee SD. Effect of chronic CB1 cannabinoid receptor antagonism on livers of rats with biliary cirrhosis. Clin Sci (Lond). 2007;112:533-542. [Cited in This Article: ] |
| 18. | Bishnoi M, Patil CS, Kumar A, Kulkarni SK. Co-administration of acetyl-11-keto-beta-boswellic acid, a specific 5-lipoxygenase inhibitor, potentiates the protective effect of COX-2 inhibitors in kainic acid-induced neurotoxicity in mice. Pharmacology. 2007;79:34-41. [Cited in This Article: ] |
| 19. | Kim JS, Kim JC, Shim SH, Lee EJ, Jin W, Bae K, Son KH, Kim HP, Kang SS, Chang HW. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch Pharm Res. 2006;29:617-623. [Cited in This Article: ] |
| 20. | Janusz JM, Young PA, Ridgeway JM, Scherz MW, Enzweiler K, Wu LI, Gan L, Chen J, Kellstein DE, Green SA. New cyclooxygenase-2/5-lipoxygenase inhibitors. 3. 7-tert-butyl-2, 3-dihydro-3,3-dimethylbenzofuran derivatives as gastrointestinal safe antiinflammatory and analgesic agents: variations at the 5 position. J Med Chem. 1998;41:3515-3529. [Cited in This Article: ] |
| 21. | Zheng M, Zhang Z, Zhu W, Liu H, Luo X, Chen K, Jiang H. Essential structural profile of a dual functional inhibitor against cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX): molecular docking and 3D-QSAR analyses on DHDMBF analogues. Bioorg Med Chem. 2006;14:3428-3437. [Cited in This Article: ] |
| 22. | Danz H, Stoyanova S, Thomet OA, Simon HU, Dannhardt G, Ulbrich H, Hamburger M. Inhibitory activity of tryptanthrin on prostaglandin and leukotriene synthesis. Planta Med. 2002;68:875-880. [Cited in This Article: ] |
| 23. | Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605-1621. [Cited in This Article: ] |
| 24. | Nieves D, Moreno JJ. Effect of arachidonic and eicosapentaenoic acid metabolism on RAW 264.7 macrophage proliferation. J Cell Physiol. 2006;208:428-434. [Cited in This Article: ] |
| 25. | Garcia Rodriguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007;132:498-506. [Cited in This Article: ] |
| 26. | Pommery J, Pommery N, Henichart JP. Modification of eicosanoid profile in human blood treated by dual COX/LOX inhibitors. Prostaglandins Leukot Essent Fatty Acids. 2005;73:411-417. [Cited in This Article: ] |
| 27. | Huang RH, Chai J, Tarnawski AS. Identification of specific genes and pathways involved in NSAIDs-induced apoptosis of human colon cancer cells. World J Gastroenterol. 2006;12:6446-6452. [Cited in This Article: ] |
| 28. | Fiorucci S, Distrutti E, de Lima OM, Romano M, Mencarelli A, Barbanti M, Palazzini E, Morelli A, Wallace JL. Relative contribution of acetylated cyclo-oxygenase (COX)-2 and 5-lipooxygenase (LOX) in regulating gastric mucosal integrity and adaptation to aspirin. FASEB J. 2003;17:1171-1173. [Cited in This Article: ] |
| 29. | Ding XZ, Tong WG, Adrian TE. Cyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancer. Pancreatology. 2001;1:291-299. [Cited in This Article: ] |
| 30. | Fiorucci S, Meli R, Bucci M, Cirino G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem Pharmacol. 2001;62:1433-1438. [Cited in This Article: ] |