Published online Apr 21, 2008. doi: 10.3748/wjg.14.2323
Revised: February 21, 2008
Published online: April 21, 2008
AIM: To evaluate the efficacy of Myelophil, an extract containing Astragali Radix and Salviae Radix, for reducing complications induced by 5-fluorouracil (5-FU) in a gastrointestinal cancer model.
METHODS: We injected 5-FU into mice and then administered Myelophil to examine the ability of the drug to treat the side effects of 5-FU in mice. Peripheral blood counts, histological examinations, and colony-forming assays of bone marrow were conducted, followed by swimming tests and assessment of survival times.
RESULTS: Myelophil restored red and white blood cells and platelets in blood, and recovered cell density in bone marrow to levels comparable to those observed within the control group. In addition, Myelophil significantly increased colony-forming unit granulocyte-macrophage (CFU-GM) and CFU-erythroid (CFU-E) compared to the control group. We confirmed that interleukin-3 gene expression was upregulated by Myelophil in spleen cells. Myelophil administration also doubled the survival rate of mice that were severely myelosuppressed as a result of 5-FU injection at a lethal dose of 70%. Finally, the swimming performance of mice significantly improved as a result of Myelophil treatment.
CONCLUSION: These results provide experimental evidence in support of clinical applications of Myelophil to minimize 5-FU-induced myelosuppression and improve general post-chemotherapy health.
- Citation: Shin JW, Lee MM, Son JY, Lee NH, Cho CK, Chung WK, Cho JH, Son CG. Myelophil, a mixture of Astragali Radix and Salviae Radix extract, moderates toxic side effects of fluorouracil in mice. World J Gastroenterol 2008; 14(15): 2323-2328
- URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2323.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2323
The occurrence of undesired effects that result from conventional chemotherapy or irradiation for cancer is inevitable. Nevertheless, reducing adverse effects is a critical issue for patients and doctors, given the importance of quality of life, as well as survival gains[1–3]. Accordingly, mitigation of chemotherapy-induced side effects and the development of novel chemotherapeutic agents with fewer toxic effects have been major focuses of recent medical investigations[45]. In particular, cancer-therapy-related fatigue, diarrhea or myelosuppression-related symptoms are closely associated with failure of the therapy itself. Therefore, many therapeutic developments, including herb-derived remedies, have focused on treating these side effects[6–9].
Fluorouracil is one of the most commonly used drugs to treat gastrointestinal cancers, including those in the stomach, colon and liver, and it commonly causes fatigue, diarrhea and sometimes myelosuppression[1011]. Myelophil is a mixture of Astragali Radix and Salviae Radix extract representing Qi and blood, respectively, which support the liver and gastrointestinal system according to theories of Oriental medicine. Astragali Radix displays immunomodulating, hematopoietic, and anti-fibrotic properties[12–14]. Salviae Radix exhibits antioxidant, antiatherosclerosis, and antiplatelet aggregation pharmaceutical effects[15–17]. We have used this drug to treat mainly gastrointestinal cancer patients with post-therapeutic complications such as leukopenia, anemia or severe fatigue since 2002.
Here, we evaluated the therapeutic efficacy of an extract mixture that contained Astragali Radix and Salviae Radix for reducing complications from cancer chemotherapy, using a 5-fluorouracil (5-FU)-induced myelosuppression mouse model.
Astragalus membranaceus (Leguminosae, VS No: AM-2006-02-Ra) and Salvia miltiorrbizae (Labiatae, VS No: SM-2006-01-Ra) were provided by Daejeon Oriental Medical College, Dunsan Oriental Hospital, of Daejeon University, identified by Professor SI Yim of Daejeon University and stored at our laboratory for future use. Samik Pharmaceutical Company (Seoul, Korea) manufactured a lyophilized aqueous extract of Myelophil (mixture of Astragali Radix and Salviae Radix; 1:1) according to over-the-counter Korean monographs. A final product with 20.52% (w/w) yield was stored for future use (VS No: MP-2006-01-WE). 5-FU was purchased from Choongwae Pharma Corporation (Seoul, Korea). Other chemicals were obtained from Sigma (St. Louis, MO, USA).
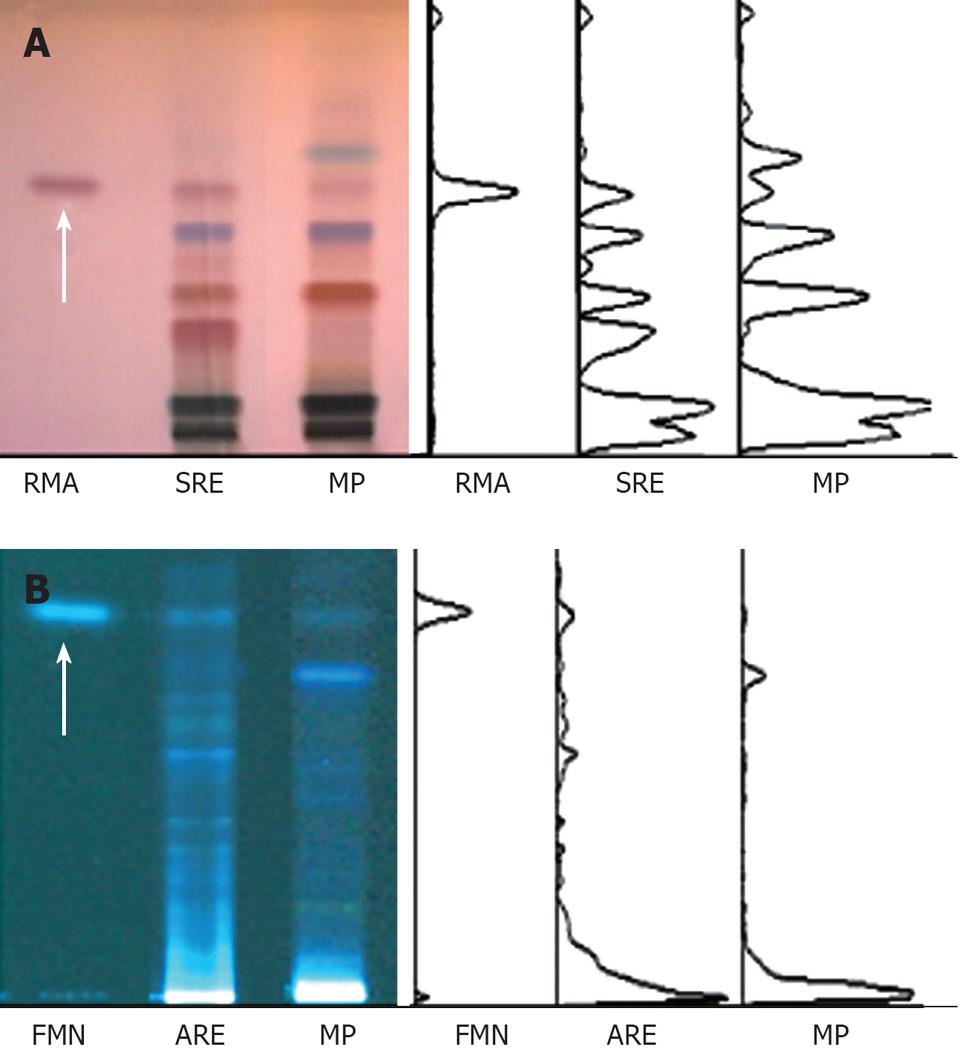
For the fingerprinting of Myelophil, the water extract of Astragali radix and Salviae radix, and their standard components, formononetin (Sigma) and rosmarinic acid (Carl Roth, Karlsruhe, Germany) were prepared for the high performance thin layer chromatography (HPTLC) system (CAMAG, Muttenz, Switzerland). They were dissolved in HPLC-grade methanol and applied to pre-washed HPTLC plate silica gel 60 F254 (Merck, Darmstadt, Germany) with an automated applicator (Linomat IV; CAMAG). The samples were then separated and the migrated components were visualized prior to or after derivatization under UV radiation at 366 nm or white light using Reprostar 3 with a digital camera (CAMAG, Figure 1).
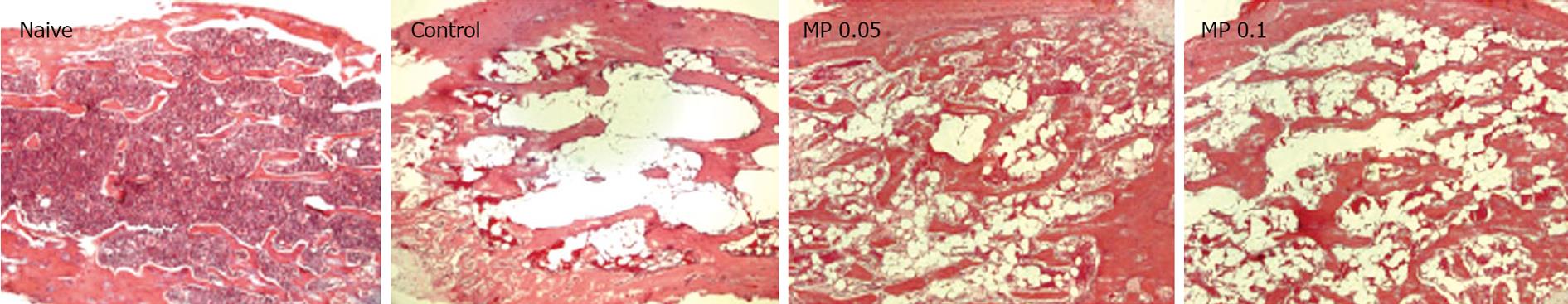
To examine the therapeutic effects of Myelophil on 5-FU-induced myelosuppression, 60 6-wk-old male ICR mice (Koatech, Gyeonggi-do, Korea) were divided into three groups (20 control, and 20 with low- and 20 with high-concentration Myelophil treatment). Another five mice were sacrificed to record physiological standards for hematological parameters at time 0. All three groups were injected intraperitoneally with 0.3 g/kg 5-FU on d 0. Beginning on d 2, Myelophil (0.05 g/kg or 0.1 g/kg) or distilled water (control group) was administered orally once daily for 10 consecutive days. Five mice per group were serially sacrificed on d 0, 4, 7, 10 and 13, and complete blood counts were analyzed using a blood cell counter (HEMAVET; CDC Technologies, CT, USA). In addition, all left-side femoral bones from the mice on d 7 were prepared for general histopathological evaluation, including fixation, decalcification, sectioning (4 &mgr;m thickness), as well as hematoxylin and eosin (HE) staining.
To directly examine the effects of Myelophil on bone marrow stem cells, C57BL/6 mice (three for each of the control, and low- and high-concentration Myelophil groups) were injected intraperitoneally with 5-FU (0.2 g/kg). Mice were given Myelophil (0.05 g/kg or 0.1 g/kg) or distilled water (for naive and control groups) for 5 consecutive days beginning 2 d after 5-FU injection. Bone marrow cells were isolated from femurs, and nucleated cells were counted using a blood cell counter (HEMAVET; CDC Technologies). After thoroughly mixing the nucleated cells (400 &mgr;L 2 × 105) with 4 mL MethoCult methylcellulose-based medium (Stem Cell Technologies, Seattle, WA, USA), media (1 mL per dish in triplicate) were cultured in a 5% CO2 incubator for 7 d. According to the morphological characteristics, the number of colonies assessed by CFU-GM or CFU-E was counted under an inverted microscope.
Splenocytes isolated from BALB/c male mice were seeded in a six-well culture plate (2.8 × 107 cells per well), and treated with or without Myelophil (0.001, 0.01 or 0.1 g/L) for 18 h. After purification of total RNA using an RNA mini kit (Qiagen, Valencia, CA, USA) and cDNA synthesis, quantitative real-time PCR was performed using SYBR Green Supermix reagent (Bio-Rad, CA, USA) according to the manufacturer's protocol. The primer sequences (forward and reverse, respectively) were as follows: β-actin, GTGGGGCGCCCCAGGCACCA and CTCCTTAATGTCACGCACGATTTC; IL-3, TACATCTGCGAATGACTCTGC and GGCTGAGGTGGTCTAGAGGTT.
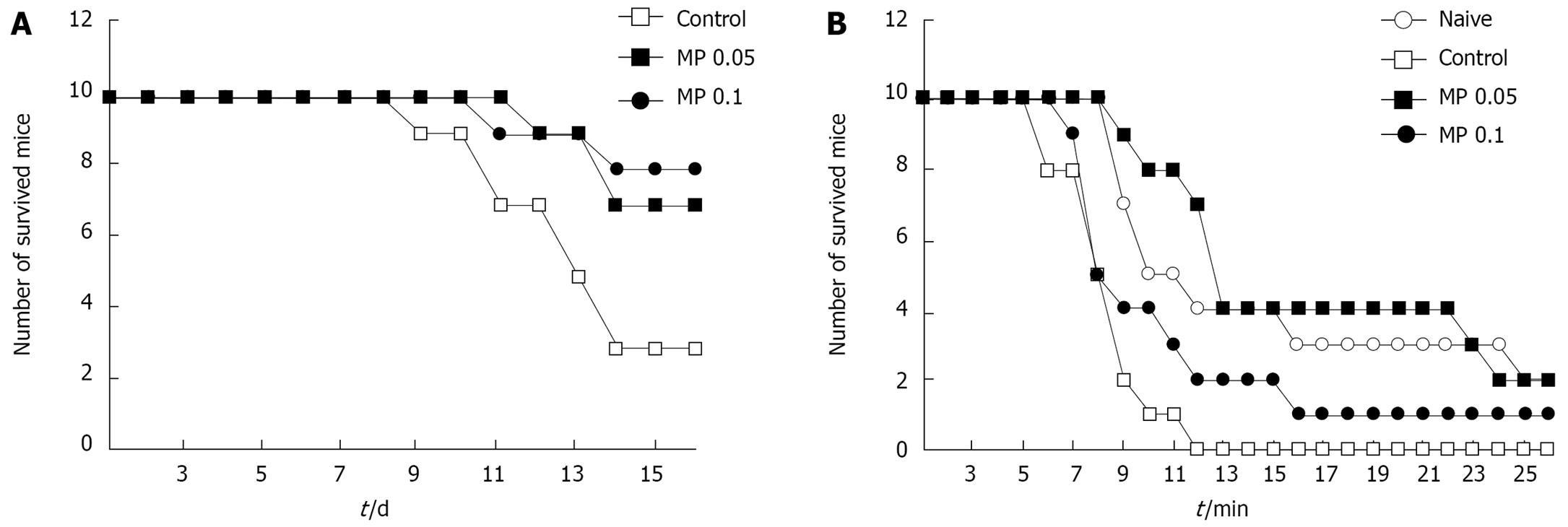
To examine whether Myelophil affected the survival time of mice with severe or moderate myelosuppression induced by 5-FU, we conducted two tests. First, severe myelosuppression was induced in 30 male ICR mice (10 in each of the control, and low- and high-concentration Myelophil groups) by 5-FU treatment (0.5 g/kg, intraperitoneally). Two days later, Myelophil (0.05 or 0.1 g/kg) or distilled water (induced group) was administered orally once daily for 10 consecutive days. The number of surviving mice was monitored for the next 20 d.
For the second test, moderate myelosuppression was induced in 30 male ICR mice (10 in each of the control, and low- and high-concentration Myelophil groups) by 5-FU treatment (0.3 g/kg, intraperitoneally). Beginning 2 d following 5-FU injection, Myelophil (0.05 or 0.1 g/kg) or distilled water (for the naïve and control groups) was given orally once daily for five consecutive days. On the final day, all mice were forced to swim in a pool with 22°C water for 30 min. Mice were monitored for survival time and swimming performance.
The results were expressed as mean ± SD. Statistical analysis of the data was conducted using Student's t test with significance levels of P < 0.05.
First, we examined changes in hematological parameters (leukocyte, erythrocyte and platelet counts) in 5-FU-induced (0.3 g/kg) myelosuppressed mice every 3 d. As shown in Table 1, peripheral blood white blood cell, platelet, and red blood cell levels drastically decreased, with the lowest numbers recorded on d 7. However, the observed pancytopenia was ameliorated by Myelophil administration, and the number of leukocytes rapidly recovered compared to that in untreated control mice (0.05 g/kg Myeolophil, P = 0.0387; 0.1 g/kg Myelophil, P = 0.0014).
| Cells/groups | d 0 | d 4 | d 7 | d 10 | d 13 | |
| WBC (103 cells/&mgr;L) | Control | 5.5 ± 1.2 | 2.3 ± 0.3 | 0.7 ± 0.2 | 2.0 ± 1.0 | 3.7 ± 1.5 |
| MP 50 | 5.5 ± 1.2 | 2.3 ± 0.9 | 1.2 ± 0.4a | 2.5 ± 0.9 | 6.4 ± 1.6a | |
| MP100 | 5.5 ± 1.2 | 2.4 ± 0.3 | 1.6 ± 0.4b | 2.9 ± 0.6 | 4.2 ± 1.0a | |
| RBC (106 cells/&mgr;L) | Control | 7.1 ± 0.8 | 7.3 ± 0.6 | 5.3 ± 0.2 | 6.3 ± 1.2 | 7.0 ± 1.0 |
| MP 50 | 7.1 ± 0.8 | 6.3 ± 0.6a | 5.9 ± 0.4b | 6.9 ± 0.7 | 6.9 ± 1.2 | |
| MP 100 | 7.1 ± 0.8 | 6.6 ± 0.5 | 6.6 ± 0.4b | 6.7 ± 0.5 | 7.2 ± 1.0 | |
| Platelets (105 cells/&mgr;L) | Control | 13.8 ± 0.8 | 8.5 ± 1.3 | 5.3 ± 1.5 | 7.3 ± 1.7 | 12.0 ± 5.3 |
| MP 50 | 13.8 ± 0.8 | 8.8 ± 1.3 | 8.2 ± 1.4a | 9.1 ± 3.2 | 17.7 ± 6.6 | |
| MP 100 | 13.8 ± 0.8 | 9.8 ± 1.8 | 9.0 ± 2.2a | 10.9 ± 4.5 | 16.2 ± 4.9 |
We examined the cellular density of femoral bone marrow from d 7 mice. Similar to the peripheral blood counts, 5-FU injection radically reduced the cellular component in bone marrow by vacuolation, and this was moderately improved by Myelophil treatment (Figure 2).
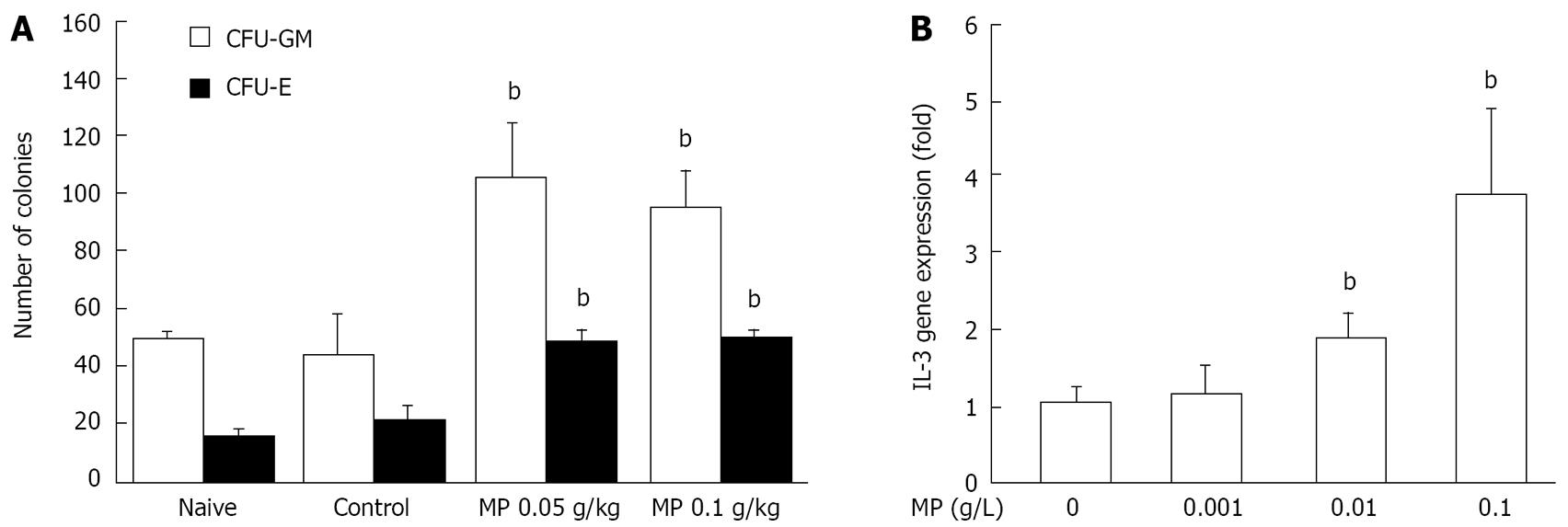
To investigate how Myelophil affected the hematopoietic stem cells, leukocyte or erythrocyte-lineage colonies were determined using colony forming assay. Myelophil treatment significantly increased the colony numbers of both leukocyte (0.05 g/kg Myeolophil, P = 0.0087; 0.1 g/kg Myelophil, P = 0.0029) and erythrocyte lineages (0.05 g/kg Myeolophil, P = 0.0018; 0.1 g/kg Myeolophil, P = 0.0021), as shown in Figure 3A.
We examined changes in IL-3 gene expression in splenocytes following co-culturing with Myelophil using RT-PCR. IL-3 expression was increased in a dose-dependent manner. At a concentration of 0.1 g/L Myelophil, this gene was up-regulated four-fold (Figure 3B).
Following injection with 0.5 g/kg 5-FU (LD70, determined in our experiments), the survival rate of the control group was 30% by 14 d, whereas in the Myelophil-treated groups it was 70%-80% (Figure 4A). Myelophil treatment significantly protected mice from loss of body weight after 5-FU injection (data not shown). In addition, Myelophil treatment extended the survival time of mice that were forced to swim in a pool after injection with 0.3 g/kg of 5-FU (Figure 4B).
We used a 5-FU-induced myelosuppression mouse model to evaluate the experimental efficacy of Myelophil, with relevance to the clinical application of reducing chemotherapy-induced side effects. 5-FU is one of the most commonly used anti-metabolitic chemotherapeutic drugs for colon, stomach, liver, and head and neck cancer. It has also been applied in anti-myelosuppressive studies due to its observed toxicity toward bone marrow[18–20]. We observed that a single injection of 0.5 g/kg 5-FU caused > 70% mortality within 20 d, whereas 0.3 g/kg 5-FU induced mild myelosuppression with no incidence of death.
Peripheral white blood cell, platelet and red blood cell levels drastically decreased in 5-FU-induced (0.3 g/kg) myelosuppressed mice. On d 7, these levels were the lowest and were in accordance with those of severe leukopenia, moderate thrombocytopenia and mild anemia. However, the observed pancytopenia was ameliorated by Myelophil administration, and the number of leukocytes rapidly recovered compared to that in untreated control mice. Next, we examined the cellular density of femoral bone marrow from d 7 mice. Similar to the peripheral blood results, 5-FU injection radically reduced the cellular component in bone marrow induced large vacuole formation, and this was moderately improved by Myelophil treatment (Figure 2). These results suggest that Myelophil might be beneficial for the alleviation of chemotherapy-associated high susceptibility to pathogenic microorganisms, which is a major problem post-treatment[2122].
Consequently, we investigated how Myelophil affected the hematopoietic stem cells via differential examination of leukocyte- or erythrocyte-lineage colonies. The number and lineage of a colony was decided mainly by quantity and quality of stem cells in different groups. Our results showed Myelophil treatment significantly increased the colony numbers of both leukocyte and erythrocyte lineages (Figure 3A). Processes of hematopoiesis are under the control of various hematopoietic growth factors, such as IL-3, erythropoietin (EPO), thrombopoietin (TPO), granulocyte colony-stimulating factor (G-CSF), or granulocyte/macrophage CSF (GM-CSF)[23]. These growth factors have lineage-specific hematopoietic functions and different cellular excretion sources[24]. Specifically, IL-3 supports proliferation and differentiation of hematopoietic stem cells, as well as various cell lineages in hematopoiesis[25], and is secreted mainly from natural killer T cells[26]. Therefore, we examined changes in IL-3 gene expression in splenocytes following co-culturing with Myelophil. The result showed that IL-3 expression was increased four-fold (Figure 3B).
Given the above results, we observed how Myelophil could restore myelosuppression via IL-3 up-regulation. Generally, myelosuppression is linked strongly to other common side effects caused by conventional cancer therapy, such as fatigue or low energy, as well as low immunity.
Myelophil-treatment significantly protected mice from loss of body weight after 5-FU (0.5 g/kg) injection (data not shown). In addition, Myelophil treatment extended the survival times of mice that were forced to swim in a pool after injection of 0.3 g/kg 5-FU (Figure 4B).
5-FU-induced myelosuppression is the dose-limiting toxicity associated with substantial life-threatening risk and life span of cancer patients[2728]. Many herbal medicines are currently being investigated as good candidates for improving quality of life and reducing toxic side effects such as myelosuppression[29–32]. One group has reported the efficacy of one of the two medicinal plant extracts in Myelophil on hematopoiesis induction in mice[14]. We found that a mixture of Astragali Radix and Salviae Radix was more effective than a single herb administered alone in our model system (data not shown). We have prescribed Myelophil to treat post-therapeutic complications such as anemia, leukopenia or severe fatigue, mainly for gastrointestinal cancer patients, according to its oriental pharmaceutical theory since 2002.
Herein, we have provided experimental evidence relevant to clinical applications of Myelophil for minimizing cancer chemotherapy-induced side-effects, using a fluorouracil-induced myelosuppression mouse model.
Anticancer-therapy-induced side effects are closely associated with life span and quality of life in cancer patients, so reducing or preventing these has been a major issue in cancer treatment. Astragali Radix and Salviae Radix extract, Myelophil, has been used to treat mainly gastrointestinal cancer patients with post-therapeutic complications such as leukopenia, anemia or severe fatigue since 2002. This study demonstrated the efficacy of Myelophil for moderating the toxic side effects of 5-FU.
Fluorouracil is one of the most commonly used drugs to treat gastrointestinal cancers, including stomach, colon and liver, but it commonly causes fatigue, diarrhea and sometimes myelosuppression. Myelophil significantly ameliorated the toxic side effects of 5-FU, such as leukopenia, anemia and thrombocytopenia and improved survival rate in myelosuppressed mice.
Myelophil showed dramatic activity against the side effects of 5-FU, a very widely used drug. This study showed evidence that a herbal remedy can be a good candidate for improving quality of life in cancer patients.
Myelophil may be of benefit to cancer patients suffering from chemotherapy-induced side effects.
This manuscript examines two herbal extract mixtures that reduce complications induced by the chemotherapeutic agent, 5-FU in a gastrointestinal cancer model. The bone marrow data, colony-forming assay, and interleukin gene expression all support the moderating effects of Myelophil on toxic side effects of 5-FU, and show the possibilities for clinical applications.
| 1. | Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155-163. [Cited in This Article: ] |
| 2. | Schuell B, Gruenberger T, Kornek GV, Dworan N, Depisch D, Lang F, Schneeweiss B, Scheithauer W. Side effects during chemotherapy predict tumour response in advanced colorectal cancer. Br J Cancer. 2005;93:744-748. [Cited in This Article: ] |
| 3. | Hassett MJ, O'Malley AJ, Pakes JR, Newhouse JP, Earle CC. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108-1117. [Cited in This Article: ] |
| 4. | Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20:4713-4721. [Cited in This Article: ] |
| 5. | Senecal FM, Yee L, Gabrail N, Charu V, Tomita D, Rossi G, Schwartzberg L. Treatment of chemotherapy-induced anemia in breast cancer: results of a randomized controlled trial of darbepoetin alfa 200 microg every 2 weeks versus epoetin alfa 40,000 U weekly. Clin Breast Cancer. 2005;6:446-454. [Cited in This Article: ] |
| 6. | Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol. 2001;28:7-14. [Cited in This Article: ] |
| 7. | Mayers C, Panzarella T, Tannock IF. Analysis of the prognostic effects of inclusion in a clinical trial and of myelosuppression on survival after adjuvant chemotherapy for breast carcinoma. Cancer. 2001;91:2246-2257. [Cited in This Article: ] |
| 8. | Steele TA. Chemotherapy-induced immunosuppression and reconstitution of immune function. Leuk Res. 2002;26:411-414. [Cited in This Article: ] |
| 9. | Son CG, Han SH, Cho JH, Shin JW, Cho CH, Lee YW, Cho CK. Induction of hemopoiesis by saenghyuldan, a mixture of Ginseng radix, Paeoniae radix alba, and Hominis placenta extracts. Acta Pharmacol Sin. 2003;24:120-126. [Cited in This Article: ] |
| 10. | Wilke HJ, Van Cutsem E. Current treatments and future perspectives in colorectal and gastric cancer. Ann Oncol. 2003;14 Suppl 2:ii49-ii55. [Cited in This Article: ] |
| 11. | Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, Francois E, Jacob JH, Levoir D, Taamma A. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002;20:4543-4548. [Cited in This Article: ] |
| 12. | Cho WC, Leung KN. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J Ethnopharmacol. 2007;113:132-141. [Cited in This Article: ] |
| 13. | Sun WY, Wei W, Wu L, Gui SY, Wang H. Effects and mechanisms of extract from Paeonia lactiflora and Astragalus membranaceus on liver fibrosis induced by carbon tetrachloride in rats. J Ethnopharmacol. 2007;112:514-523. [Cited in This Article: ] |
| 14. | Zhu XL, Zhu BD. Mechanisms by which Astragalus membra-naceus injection regulates hematopoiesis in myelosuppressed mice. Phytother Res. 2007;21:663-667. [Cited in This Article: ] |
| 15. | Tang MK, Ren DC, Zhang JT, Du GH. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine. 2002;9:405-409. [Cited in This Article: ] |
| 16. | Koo BS, Kwon TS, Kim CH. Salviae miltiorrhizae radix inhibits superoxide generation by activated rat microglias and mimics the action of amphetamine on in vitro rat striatal dopamine release. Neurochem Res. 2004;29:1837-1845. [Cited in This Article: ] |
| 17. | Li S, Wan L. Experimental study on the preventive mechanism of salviae miltiorrhizae against atherosclerosis in rabbits models. J Huazhong Univ Sci Technolog Med Sci. 2004;24:233-235. [Cited in This Article: ] |
| 18. | Kojima S, Takaba K, Kimoto N, Takeda T, Kakuni M, Mizutani M, Suzuki K, Sato H, Hara T. Protective effects of glutathione on 5-fluorouracil-induced myelosuppression in mice. Arch Toxicol. 2003;77:285-290. [Cited in This Article: ] |
| 19. | Takano F, Tanaka T, Aoi J, Yahagi N, Fushiya S. Protective effect of (+)-catechin against 5-fluorouracil-induced myelo-suppression in mice. Toxicology. 2004;201:133-142. [Cited in This Article: ] |
| 20. | Vento S, Cainelli F. Infections in patients with cancer undergoing chemotherapy: aetiology, prevention, and treatment. Lancet Oncol. 2003;4:595-604. [Cited in This Article: ] |
| 21. | Rolston KV. Challenges in the treatment of infections caused by gram-positive and gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;40 Suppl 4:S246-S252. [Cited in This Article: ] |
| 22. | Savino W, Smaniotto S, Dardenne M. Hematopoiesis. Adv Exp Med Biol. 2005;567:167-185. [Cited in This Article: ] |
| 23. | Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034-2045. [Cited in This Article: ] |
| 24. | Mangi MH, Newland AC. Interleukin-3 in hematology and oncology: current state of knowledge and future directions. Cytokines Cell Mol Ther. 1999;5:87-95. [Cited in This Article: ] |
| 25. | Leite-de-Moraes MC, Lisbonne M, Arnould A, Machavoine F, Herbelin A, Dy M, Schneider E. Ligand-activated natural killer T lymphocytes promptly produce IL-3 and GM-CSF in vivo: relevance to peripheral myeloid recruitment. Eur J Immunol. 2002;32:1897-1904. [Cited in This Article: ] |
| 26. | Cella D. Factors influencing quality of life in cancer patients: anemia and fatigue. Semin Oncol. 1998;25:43-46. [Cited in This Article: ] |
| 27. | Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616-1634. [Cited in This Article: ] |
| 28. | Biran H, Sulkes A, Biran S. 5-Fluorouracil, doxorubicin (adriamycin) and mitomycin-C (FAM) in advanced gastric cancer: observations on response, patient characteristics, myelosuppression and delivered dosage. Oncology. 1989;46:83-87. [Cited in This Article: ] |
| 29. | Zhang M, Liu X, Li J, He L, Tripathy D. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane Database Syst Rev. 2007;46:CD004921. [Cited in This Article: ] |
| 30. | Liao HF, Chen YJ, Yang YC. A novel polysaccharide of black soybean promotes myelopoiesis and reconstitutes bone marrow after 5-flurouracil- and irradiation-induced myelosuppression. Life Sci. 2005;77:400-413. [Cited in This Article: ] |
| 31. | Sugiyama K, Ueda H, Ichio Y. Protective effect of juzen-taiho-to against carboplatin-induced toxic side effects in mice. Biol Pharm Bull. 1995;18:544-548. [Cited in This Article: ] |