Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.895
Revised: November 10, 2006
Accepted: January 10, 2007
Published online: February 14, 2007
AIM: To assess whether radiation dose and duration of treatment influence local control and survival of patients with locally advanced anal cancer treated with definitive chemoradiation.
METHODS: Twenty-eight consecutive patients who were treated with definitive radiation therapy for bulky anal cancers (> 5 cm in size) were reviewed. Nineteen patients had T3 lesions, 8 patients had T4 lesions, and 15 patients had lymph node involvement. The median tumor size was 7.5 cm. All but one patient received concurrent chemoradiation. The median radiation dose was 54 Gy. The median duration of treatment was 58 d.
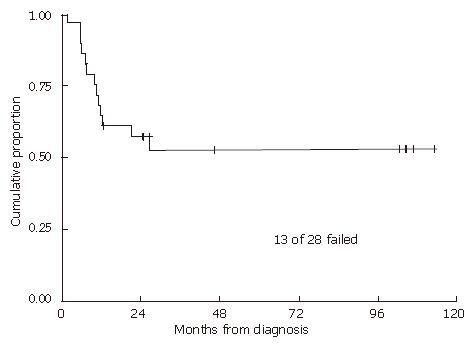
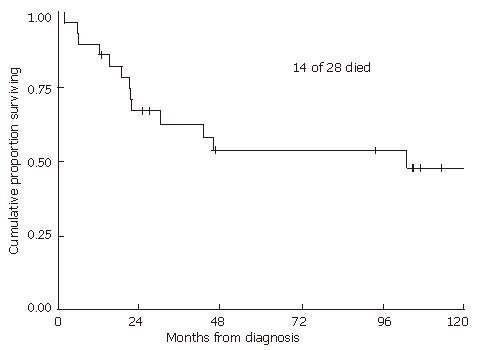
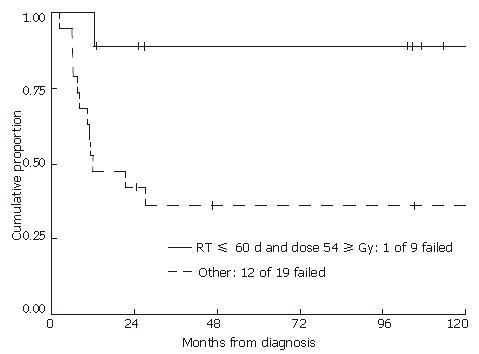
RESULTS: With a median follow-up of 2.5 years in all patients and 7.8 years in living patients, the 2-year local recurrence-free probability was 57% and overall survival rate was 67%. Neither radiation dose nor duration of treatment alone was predictive of either time to local failure or overall survival. However, longer treatment breaks can potentially mask an advantage over higher radiation doses. Therefore, we examined those patients who received ≥ 54 Gy within 60 d, comparing them to the rest of the patients. Of patients who received ≥ 54 Gy within 60 d, local progression-free probability was 89% versus 42% for the rest of the group (P = 0.01).
CONCLUSION: Local failure is a significant problem in locally advanced carcinomas of the anal canal. Higher radiation doses with limited treatment breaks may offer an increase in local control and survival.
- Citation: Huang K, Haas-Kogan D, Weinberg V, Krieg R. Higher radiation dose with a shorter treatment duration improves outcome for locally advanced carcinoma of anal canal. World J Gastroenterol 2007; 13(6): 895-900
- URL: https://www.wjgnet.com/1007-9327/full/v13/i6/895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.895
Combined chemoradiation has been established as the standard treatment for epidermoid anal cancer by randomized trials that have demonstrated improvements in local control and disease free survival compared to radiation alone[1,2]. Administered with radiation, the combination of 5-flurouracil (5-FU) and mitomycin C is the regimen of choice, providing superior disease-free survival compared to 5-FU alone[3]. However, for locally advanced anal cancer, especially lesions greater than 5 cm, local control rates of only 50% have been achieved[4,5]. Higher radiation dose has been shown to improve outcome in some studies[6-9]. A phase II radiation dose escalation trial for anal cancer performed by the Radiation Therapy Oncology Group (RTOG) showed increased colostomy rates with increased doses compared to patients treated with a lower dose in a previous RTOG trial[10]. The worse outcome may in part be attributed to a mandatory 2 wk break in this trial. It is unclear if increasing the radiation dose in patients with locally advanced anal cancer receiving combined modality therapy will improve the results compared with doses of 45-50 Gy[10].
In order to assess whether higher radiation doses improve clinical outcome for patients with locally advanced anal cancer, we retrospectively reviewed treatment outcomes of patients with bulky (> 5 cm) epidermoid anal cancer. Radiation dose, treatment time, as well as patient, tumor and treatment related factors were evaluated for prognosis.
This work has been approved by the Institutional Review Board of the University of California, San Francisco.Between 1982 and 2000, 28 consecutively treated patients underwent definitive radiation therapy for documented bulky anal cancers (disease greater than 5 cm in primary tumor or lymph nodes). Patients who had local excision prior to radiotherapy were excluded. Of 28 patients, 27 had bulky disease at primary sites and one had bulky lymph node disease. One patient was treated for bulky recurrent disease after abdominoperineal resection.
One patient declined treatment with chemotherapy and was treated with radiation alone, while the other 27 patients received concurrent chemoradiation. Chemotherapy consisted of 5-FU and mitomycin C in 23 patients, 5-FU and cisplatin in one patient and 5-FU alone in three patients. 5-FU was given at 1000 mg/m2 over 4 d. Generally mitomycin C was given at 10 mg/m2. Cisplatin was given at 75 mg/m2 every 4 wk. One patient was treated with induction 5-FU and cisplatin with progressive disease, followed by 5-FU and mitomycin C concurrently with radiation. All patients received 2 cycles of chemotherapy generally at an interval of 4 wk concurrently with radiation, except for one patient who died of fulminant hepatitis after receiving only one cycle of chemotherapy. Two patients received 5-FU alone during the second cycle after severe hematologic toxicity associated with the first cycle of chemotherapy with 5-FU and mitomycin.
Radiation was administered utilizing 6 or 18 MV photons. Treatment fields were AP/PA to the whole pelvis and bilateral inguinal regions, followed by a lateral field boost plus/minus electron field boost to cover the inguinal regions. The first field reduction was at 30.6 Gy to the true pelvis, followed by subsequent field reductions at 36 Gy and 45 Gy. For patients who had a CD4 count < 200, the initial field was the true pelvis to minimize toxicity. Before 1985, radiation doses of 40-50 Gy were planned, while after 1985, target radiation doses were increased to 54-60 Gy. Five patients did not receive the planned doses of radiation, secondary to toxicity in 3 patients, receiving 36 Gy, 50.4 Gy and 51.4 Gy, respectively, and noncompliant in 2 patients, receiving 50.4 Gy and 45 Gy, respectively. The radiation doses ranged from 31 to 65 Gy, with a median of 54 Gy. The dose per fraction ranged from 1.6 to 2.0 Gy once daily. The lower dose per fraction of 1.6 to 2.0 Gy was used in the early 80’s when radiation was first used concurrently with chemotherapy.
All the patients were evaluated at least once a week during radiotherapy. The patients were then evaluated every 1-2 mo for the first 6 mo, followed by every 3 mo for the next 6-12 mo, every 4-6 mo from 18 mo through 3 years, and annually thereafter. At each follow-up visit, a physical examination, including digital rectal examination, and palpation of the inguinal regions was performed. Acute and late effects on normal tissues were graded according to the CTCAE v3 scoring criteria.
This is a retrospective analysis of treatment outcomes for bulky anal carcinoma treated at UCSF. Descriptive statistics (mean, median, and proportions) were calculated to characterize the patient, disease, and treatment features. Estimates of survival rates and recurrence-free probabilities were calculated using the Kaplan-Meier product limit method. Durations were calculated from the date of diagnosis. Univariate analyses were performed to evaluate factors that may be predictors of outcome. The log rank test was used to compare distributions of subsets.
The cohort included 10 women and 18 men with a median age of 63 years (range 30-79 years). Eight patients had cloacgenic carcinoma and 20 patients had squamous cell carcinoma. Table 1 shows the T- and N-stage distributions of the patients according to the 2002 AJCC staging classification. Nineteen patients had T3 lesions, 8 patients had T4 lesions, 1 had recurrent disease and 15 patients had pathologically involved lymph nodes. The median tumor size was 7.5 (range 5.5-12) cm. Four patients were human immunodeficiency virus (HIV) positive with CD4 counts of 63 180 238 and 347, respectively.
| Stage | N0 | N1 | N2 | N3 | Total |
| T3 | 6 | 3 | 6 | 4 | 19 |
| T4 | 6 | 0 | 1 | 1 | 8 |
| Total | 12 | 3 | 7 | 5 | 27 |
With a median follow-up of 2.5 (range 0.2-17.3) years for all patients and 7.8 (range 1.1-17.3) years for living patients, the 2-year and 5-year local recurrence-free probabilities and 95% confidence intervals (CI) were 57% (95% CI: 37%-73%) and 52% (95% CI: 32%-69%), respectively (Figure 1). The 2- and 5-year overall survival estimates were 67% (95% CI: 46%-81%) and 54% (33%-71%), respectively (Figure 2). The 2- and 5-year colostomy-free survival estimates were 42% (95% CI: 24%-60%) and 33% (95% CI: 16%-51%), respectively. Sixteen patients had persistent abnormalities at the completion of radiotherapy. In 12 of the 16 patients, biopsies of the persistent abnormalities revealed pathological documentation of residue disease in 5 patients. The single patient with a bulky lymph node experienced a lymph node failure, with a concurrent distant failure 25 mo after diagnosis. In the 4 patients who were HIV positive, 2 experienced local failures, 1 experienced a regional failure, and one died of fulminant hepatitis during treatment after receiving only 36 Gy, presumably secondary to chemotherapy. In patients treated with 5-FU alone with concurrent radiation, 2 out of 3 experienced local failures. The patient who did not receive any chemotherapy also had a local recurrence.
Acute side effects of radiation therapy and chemotherapy were moderate to severe. Twenty patients had acute grade 3 or 4 toxicities including 15 patients with grade 3 dermatological toxicities, 1 patient with grade 3 gastrointestinal toxicity, and 5 patients with grade 3 and 4 with grade 4 hematologic toxicities. The worst acute toxicity was dermatological in 10 patients, hematologic in 5 patients, both dermatological and hematologic in 4 patients, and gastrointestinal in 1 patient. In patients who received ≥ 54 Gy, 15 out of 17 patients experienced grade 3 or 4 toxicity, compared to 5 out of 11 patients who received less than 54 Gy. In the 4 patients who were HIV positive, one did not have any grade 3 or 4 acute toxicity, one had grade 3 dermatological toxicity, 1 had grade 3 dermatological and hematologic toxicities, 1 had grade 3 dermatological toxicity and grade 4 hematologic toxicity and died of fulminant hepatitis after receiving 30 Gy of radiation.
The median duration of treatment was 58 (range 31-112) d. In patients who received ≥ 54 Gy, the median duration of treatment was 60 d compared to 44 d in those who received < 54 Gy. Twenty-two patients had treatment breaks longer than 3 d. Ten patients had only one treatment break and 12 had more than 1 treatment break. The median length of total treatment breaks was 11 (range 0-53) d. Twelve patients had treatment breaks totaling longer than 14 d. The median dose at the first treatment break was 30.6 (range 3.6-52.2) Gy. Three patients had treatment breaks due to non-compliance, and the rest of patients due to toxicity. For patients who received ≥ 54 Gy, 16 out of 17 patients had a break longer than 3 d, compared to 6 out of 11 patients who received < 54 Gy.
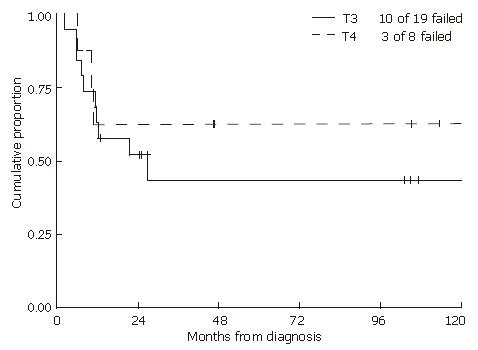
Univariate analyses revealed that male gender and HIV infection were each associated with worse overall survival (P = 0.02 and P = 0.01, respectively). Excluding HIV positive patients, male gender demonstrated a trend towards worse survival (P = 0.08). There was no significant difference in local control (Figure 3) or survival when comparing patients with T3 and T4 disease. Nodal stage, tumor size and type of chemotherapy were not predictive of either time to failure or overall survival. Neither radiation dose nor duration of treatment alone was statistically associated with either time to failure or overall survival.
Even though radiation dose or duration of treatment alone was not statistically correlated with either time to failure or overall survival, these two factors were interrelated. Patients who received ≥ 54 Gy were more likely to experience treatment breaks resulting in longer treatment duration, potentially masking an advantage over higher radiation doses. Therefore, we examined those patients who received ≥ 54 Gy within 60 d, and compared them to the rest of the group (Figure 4). Patients who received ≥ 54 Gy within 60 d had significantly improved local progression-free and overall survival rates compared to those whose treatments spanned longer than 60 d or who received less than 54 Gy (2-year local progression-free and overall survival probabilities of 89% (95% CI: 43%-98%) and 100% for treatments within 60 d compared to 42% (95% CI: 20%-62%) and 53% (95% CI: 29%-72%) for the rest of the group (P = 0.01 and P = 0.02, respectively). Of patients who received ≥ 54 Gy, 8 out of 9 patients were locally controlled if the treatment was completed within 60 d. However, for those patients who received ≥ 54 Gy with treatment durations longer than 60 d, only 2 out of 8 were locally controlled. For those patients who received less than 54 Gy, only 5 out of 11 were locally controlled (Table 2). The mean total dose in patients who received less than 54 Gy was 46 Gy.
| Fraction of patientscontrolled locally | High dose (> 54 Gy) | Low dose (< 54 Gy) |
| Short duration ( ≤ 60 d) | 8/9b | 4/9 |
| Long duration (> 60 d) | 2/8 | 1/2 |
In the current study, neither total radiation dose nor treatment time alone was prognostic for local control or survival. The two factors were interrelated in that it took a longer treatment time to deliver an overall higher radiation dose: 35 d for 45 Gy and 45 d for 60 Gy. In addition, higher radiation doses resulted in higher toxicity rates and therefore a higher likelihood of treatment breaks. In this study, patients who received ≥ 54 Gy within 60 d had significantly improved local progression-free (Figure 4) and overall survival compared to those whose treatment spanned longer than 60 d or who received less than 54 Gy. This confirms a dose response in the treatment of locally advanced anal cancer with chemoradiation, although prolonged treatment breaks negate the advantage of higher dose.
Total radiation dose has been shown to impact outcome in anal carcinoma treated with combined chemoradiation[6-9]. Constantinou et al[6] found that radiation doses of ≥ 54 Gy were associated with significantly improved survival (84% vs 47%) and local control (77% vs 61%) in anal cancer patients treated with chemoradiation. About 30% of patients in their study had T3 or T4 lesions. In a group of patients treated with chemoradiation using continuous infusion of 5-FU, local control increased from 50% in those receiving < 45 Gy, to 73% in those receiving 50-54 Gy and 83% in those receiving > 60 Gy[9]. Nigh et al[8] reported a dose response with improved local control at higher doses: 64% at < 45 Gy, 77% at 45-55 Gy and 92% at > 55 Gy. At MD Anderson, local control was 50% for all stages receiving 45-49 Gy and 90% for those patients receiving greater than or equal to 55 Gy[7].
Overall treatment time has similarly been shown to impact outcome in treatment of anal cancer[10-15]. In the second phase of the dose escalation study RTOG 9208 without the mandatory break, the 1-year colostomy rate decreased from 23% to 11%[10,14]. In an European Organization for Research and Treatment of Cancer (EORTC) trial with a similar design to RTOG 9208 including a 2 wk break at 36 Gy (but slightly different chemotherapy with 5-FU given continuously during radiation), the 3-year local control and colostomy-free interval were 88% and 81% respectively, which compared favorably to the earlier EORTC randomized trial with a 6 wk break and mitomycin C given only on the first day of radiation[12]. Built in breaks > 37.5 d correlate with poorer loco-regional control in patients treated with a median of 40 Gy to the pelvis and a 20 Gy boost with either external beam radiation or brachytherapy[15]. Ceresoli et al[13] found that overall treatment times longer than 70 d were related to a worse disease-free survival in a group of patients treated with a median radiation dose of 56 Gy. Overall treatment times > 75 d were associated with poorer local control (69% vs 85%) in a study by Allal[11].
Increased toxicities have been observed in patients treated with higher radiation doses. In the second phase of the dose escalation study RTOG 9208 without the mandatory break, 50% of patients required treatment interruptions lasting at least 7 d. In our study, 71% of patients had treatment breaks longer than 3 d. Fourteen patients had grade 3 or greater dermatological toxicities requiring treatment breaks. One way to decrease toxicities is to use conformal radiation therapy. Indeed, one study showed that conformal radiotherapy enhanced tolerance to treatment, shortened treatment time to 6 wk and significantly decreased acute toxicities[16]. In another study, intensity-modulated radiotherapy (IMRT) improved conformality and reduced normal structure dose without compromising local control in the treatment of anal cancer[17]. We have investigated the potential to improve coverage of tumor volumes while maximizing sparing of normal tissues such as the skin and bowel using IMRT and have adopted IMRT as the preferred treatment technique for locally advanced anal cancer. Grade 3 or greater hematologic toxicity was observed in 9 patients. Cisplatin-based combined modality therapy may generate less acute toxicity and a wider therapeutic index. In a retrospective analysis of patients treated with 55 Gy of radiation and continuous infusion of cisplatin and 5-FU, local control and survival were comparable to the best results reported with mitomycin C and 5-FU, with greater than 90% of patients completing treatment without significant treatment interruptions[18].
Hoffman et al[19] previously reported our institutional experience of treating HIV positive anal cancer patients with chemoradiation. Excellent disease control with acceptable morbidity was achieved in patients with a CD4 count of greater than or equal to 200[19]. There were 4 HIV positive patients with locally advanced disease in the group reported herein. Their disease control and survival were poor and toxicities were significant, although the small number of patients precluded firm conclusions regarding the role played by their HIV status.
The ideal chemotherapy regimen has yet to be established. An Eastern Oncology Group trial tested concurrent cisplatin, 5-FU, and 59.4 Gy of radiation with a 2-wk break after 36 Gy, which resulted in a complete response rate of 68%[20]. Adjuvant chemotherapy after combined chemoradiation is being tested in the ACT IIrandomized trial from the United Kingdom[21]. The recently closed randomized trial RTOG 9811 compares concurrent chemoradiation with 5-FU and mitomycin C versus induction chemotherapy and concurrent chemoradiation with 5-FU and cisplatin. In addition, for T3, T4, node positive and any lesions with residual disease after 45 Gy, a boost of 10-14 Gy is included to achieve total doses of 55-59 Gy. The result from this trial may answer some of the questions pertaining to the best treatment regimen for anal cancer.
In conclusion, local failure is a significant problem in locally advanced carcinomas of the anal canal. Although the number of patients in this study was relatively small, we believe that higher radiation doses with limited treatment breaks may offer an increase in local control and survival.
There is controversy in the field regarding whether higher radiation doses improve outcome in anal cancer. Local control rate for locally advanced anal cancer is around 50%, which clearly needs to be improved. This paper tries to address the interrelation of time and dose and generates the hypothesis of higher radiation doses with shorter treatment duration improve outcome in locally advance anal cancer.
The current focus of research is in the delivery of intensity modulated radiation therapy to treat anal cancer. The highly conformal dose distribution allows more sparing of normal tissues and high dose to the tumor, therefore, fewer toxicities and treatment breaks and hopefully improved tumor control.
Constantinou et al found that radiation doses of ≥ 54 Gy were associated with significantly improved survival (84% vs 47%) and local control (77% vs 61%) in anal cancer patients treated with chemoradiation. Other studies addressed treatment time to some extent. This is the only study looking at radiation doses and treatment time in locally advanced anal cancer.
To achieve improved local control in locally advanced anal cancer patients, radiation doses of at least 54 Gy should be given concurrently with chemotherapy. Treatment breaks should be minimized to realize the effect of the higher doses of radiation. Modern techniques such as intensity-modulated radiotherapy may be a means to deliver higher doses of radiation while minimizing toxicities and treatment breaks.
Total radiation dose is the final dose that is delivered to the gross tumor. Overall treatment time is the entire duration of radiation from beginning to end including any mandatory or elective treatment breaks. Intensity-modulated radiotherapy is a technique which delivers radiation in multiple fields of varying radiation intensity so that the dose distribution can be highly conformal around the target and minimizing doses to nearby critical tissues.
The paper addresses one of the controversies in the field, that is, whether higher radiation dose improves outcome in locally advanced anal cancer. The data suggests higher radiation dose with shorter treatment duration improves outcome in this group of patients.
S- Editor Liu Y L- Editor Zhu LH E- Editor Lu W
| 1. | Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M, Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040-2049. [PubMed] [Cited in This Article: ] |
| 2. | Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 824] [Cited by in F6Publishing: 688] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, Quivey J, Rotman M, Kerman H, Coia L. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527-2539. [PubMed] [Cited in This Article: ] |
| 4. | Gerard JP, Ayzac L, Hun D, Romestaing P, Coquard R, Ardiet JM, Mornex F. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother Oncol. 1998;46:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Peiffert D, Bey P, Pernot M, Guillemin F, Luporsi E, Hoffstetter S, Aletti P, Boissel P, Bigard MA, Dartois D. Conservative treatment by irradiation of epidermoid cancers of the anal canal: prognostic factors of tumoral control and complications. Int J Radiat Oncol Biol Phys. 1997;37:313-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Constantinou EC, Daly W, Fung CY, Willett CG, Kaufman DS, DeLaney TF. Time-dose considerations in the treatment of anal cancer. Int J Radiat Oncol Biol Phys. 1997;39:651-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Hughes LL, Rich TA, Delclos L, Ajani JA, Martin RG. Radiotherapy for anal cancer: experience from 1979-1987. Int J Radiat Oncol Biol Phys. 1989;17:1153-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Nigh SS, Smalley SR, Elman AJ, Paradelo JC, Kooser JA, Reddi R, Evans RG. Conservative Therapy for Anal Carcinoma: an Analysis of Prognostic Factors. Int J Radiat Oncol Biol Phys. 1991;21:224. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Rich TA, Ajani JA, Morrison WH, Ota D, Levin B. Chemoradiation therapy for anal cancer: radiation plus continuous infusion of 5-fluorouracil with or without cisplatin. Radiother Oncol. 1993;27:209-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 121] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | John M, Pajak T, Flam M, Hoffman J, Markoe A, Wolkov H, Paris K. Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92-08. Cancer J Sci Am. 1996;2:205-211. [PubMed] [Cited in This Article: ] |
| 11. | Allal AS, Mermillod B, Roth AD, Marti MC, Kurtz JM. The impact of treatment factors on local control in T2-T3 anal carcinomas treated by radiotherapy with or without chemotherapy. Cancer. 1997;79:2329-2335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Bosset JF, Roelofsen F, Morgan DA, Budach V, Coucke P, Jager JJ, Van der Steen-Banasik E, Trivière N, Stüben G, Puyraveau M. Shortened irradiation scheme, continuous infusion of 5-fluorouracil and fractionation of mitomycin C in locally advanced anal carcinomas. Results of a phase II study of the European Organization for Research and Treatment of Cancer. Radiotherapy and Gastrointestinal Cooperative Groups. Eur J Cancer. 2003;39:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Ceresoli GL, Ferreri AJ, Cordio S, Villa E. Role of dose intensity in conservative treatment of anal canal carcinoma. Report of 35 cases. Oncology. 1998;55:525-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | John M, Pajak T, Krieg R, Pinover WH, Myerson R. Dose Escalation without Split-course in Chemoradiation for Anal Cancer: Results of a Phase II RTOG Study. Int J Radiat Oncol Biol Phys. 1997;39:203. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Weber DC, Kurtz JM, Allal AS. The impact of gap duration on local control in anal canal carcinoma treated by split-course radiotherapy and concomitant chemotherapy. Int J Radiat Oncol Biol Phys. 2001;50:675-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Vuong T, Devic S, Belliveau P, Muanza T, Hegyi G. Contribution of conformal therapy in the treatment of anal canal carcinoma with combined chemotherapy and radiotherapy: results of a phase II study. Int J Radiat Oncol Biol Phys. 2003;56:823-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Milano MT, Jani AB, Farrey KJ, Rash C, Heimann R, Chmura SJ. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2005;63:354-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Hung A, Crane C, Delclos M, Ballo M, Ajani J, Lin E, Feig B, Skibber J, Janjan N. Cisplatin-based combined modality therapy for anal carcinoma: a wider therapeutic index. Cancer. 2003;97:1195-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Hoffman R, Welton ML, Klencke B, Weinberg V, Krieg R. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol Biol Phys. 1999;44:127-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Martenson JA, Lipsitz SR, Wagner H, Kaplan EH, Otteman LA, Schuchter LM, Mansour EG, Talamonti MS, Benson AB. Initial results of a phase II trial of high dose radiation therapy, 5-fluorouracil, and cisplatin for patients with anal cancer (E4292): an Eastern Cooperative Oncology Group study. Int J Radiat Oncol Biol Phys. 1996;35:745-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |