Published online Feb 7, 2007. doi: 10.3748/wjg.v13.i5.732
Revised: December 3, 2006
Accepted: December 21, 2006
Published online: February 7, 2007
AIM: To evaluate the efficacy and safety of tegaserod, 6 mg twice daily (b.i.d.), in men and women with chronic constipation (CC) from China.
METHODS: This was a multicenter, double-blind, placebo-controlled study. Following a 2-wk treatment-free baseline period, patients were randomized to receive either tegaserod (6 mg b.i.d.) or placebo (b.i.d.) for 4 wk. An analysis of covariance with repeated measures was used to determine the overall effect of treatment for the primary efficacy variable; the change from baseline in the number of complete spontaneous bowel movements (CSBMs) during the 4-wk treatment period. Secondary efficacy endpoints included other measures of response in terms of CSBMs, and patients’ daily and weekly assessment of bowel habits. Safety was also assessed, based on the incidence and severity of adverse events (AEs).
RESULTS: A total of 607 patients were randomized to receive either tegaserod (n = 304) or placebo (n = 303). Tegaserod treatment resulted in a rapid and significant increase from baseline in the adjusted mean number of CSBMs per week over wk 1-4 compared with placebo (1.39 vs 0.91, P = 0.0002). A statistically significant difference in favor of tegaserod was also observed for a mean increase ≥ 1 CSBM/wk over wk 1-4 (47.7% vs 35.0%, tegaserod vs placebo, respectively, P = 0.0018) and for the absolute number of ≥ 3 CSBMs/wk over wk 1-4 (25.0% vs 14.5%, tegaserod vs placebo, respectively, P = 0.0021). Improvements in other symptoms of CC were also seen in the tegaserod group, including improved stool form and reduced straining. In addition, more patients in the tegaserod group reported satisfactory relief from their constipation symptoms. The frequency and severity of AEs was comparable between tegaserod and placebo groups, with the exception of a greater incidence of diarrhea in patients receiving tegaserod (3.6%) compared with placebo (1.7%).
CONCLUSION: Tegaserod treatment improved multiple symptoms of CC and was associated with a favorable safety profile.
- Citation: Lin SR, Ke MY, Luo JY, Yuan YZ, Wang JY, diTommaso S, Walter V, Huang J. A randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of tegaserod in patients from China with chronic constipation. World J Gastroenterol 2007; 13(5): 732-739
- URL: https://www.wjgnet.com/1007-9327/full/v13/i5/732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i5.732
Chronic constipation/chronic idiopathic constipation (CC) is a gastrointestinal (GI) motility and sensory disorder that is commonly reported in many regions of the world, including Asia[1], North America[2], and Europe[3]. An epidemiological study conducted in Beijing concluded that 6.1% of the adult population were suffering from the symptoms of CC[4]. This compares with a survey of 3282 people in Hong Kong, of whom 14% were deemed to be suffering from CC[1]. The disorder is more common in women and elderly people[5]. The symptoms of CC impact on patients’ quality of life (QoL) and result in frequent visits to physicians, particularly by older patients[1,6]. In Hong Kong, approximately 25% (n = 820) of those surveyed were reported to visit a physician as a result of their symptoms[1].
The diagnosis of CC has primarily centered on the infrequency of the patients’ bowel movements (BMs). However, CC is also associated with other symptoms that include straining, hard stools, feelings of incomplete evacuation, abdominal bloating, and abdominal discomfort/pain. While the pathophysiology of CC is still unclear, a proportion of patients with CC are assumed to have impaired GI motility[7]. This prolongs the length of time that stools remain in the bowel, allowing increased absorption of water from the stools which become hard and difficult to pass. Rome II criteria refined the diagnosis of CC by providing a consensus definition that is frequently used in clinical research, and can serve as a useful guide for physicians[8]. The Rome II criteria combine symptoms of straining, stool form and feelings of incomplete evacuation with measures of bowel frequency (less than three BMs per week).
A systematic review concluded that there were too few well-designed, randomized, placebo-controlled trials to support the efficacy of many of the available treatments for CC such as bulking laxatives (e.g., psyllium), osmotic laxatives [lactulose or polyethylene glycol (PEG)], and stimulant laxatives (senna or bisacodyl)[2]. Furthermore, other symptoms can be aggravated by laxative treatment (e.g., bloating)[9]. A further review found good evidence for the efficacy of PEG and tegaserod (Grade A recommendation) and moderate evidence to support the efficacy of lactulose and psyllium (Grade B recommendation)[10]. Other treatments for CC include the modification of patients’ eating habits (increasing the consumption of dietary fiber/bulking agents), biofeedback training (where patients are taught relaxation and defecation techniques), and in severe cases, surgery. Evidence for the efficacy of these agents, however, is limited[7,11].
Targeting the pathophysiological basis of CC by stimulating intestinal motility and secretion may be a more appropriate approach for the treatment of the disorder, rather than using conventional treatments. Tegaserod is a selective agonist at the serotonin receptor, 5-HT4, and has been shown to augment the release of neurotransmitters from the enteric nerves, hence stimulating intestinal peristalsis and secretion[12,13]. Two pivotal, randomized, placebo-controlled trials have demonstrated that tegaserod [2 mg or 6 mg twice daily (b.i.d.)] effectively treats the multiple symptoms of CC[14,15]. Tegaserod also effectively relieves the multiple symptoms of patients with irritable bowel syndrome (IBS) who suffer from constipation[16-18]. The majority of patients in the pivotal CC studies were Caucasian. Given that CC is a common disorder in China, the aim of the current study was to evaluate the efficacy and safety of tegaserod in men and women with CC from China.
This was a randomized, multicenter, placebo-controlled, double-blind, parallel-group study, designed to evaluate the efficacy and safety of tegaserod in men and women with CC in China.
After completing an initial screening phase of up to 28 d, patients entered a 2-wk baseline period without study medication. At the end of the baseline period, eligible patients were randomized to receive either tegaserod 6 mg b.i.d. or placebo for 4 wk using a randomization list generated by Novartis Drug Supply Management, using Almedica Drug Label System, version 5.3 a, Almedica Technology Group Inc. All Novartis staff, other than the Drug Supply Management and the Biostatistics Quality Assurance Group, remained blind to the allocation of treatment until database lock. Randomization was performed in blocks using a 1:1 ratio. The randomization list was reviewed and locked by the Biostatistics Quality Assurance group. The identity of the treatments was concealed by using study tablets that were identical in appearance. The study was conducted in accordance with the Declaration of Helsinki and was reviewed by the Independent Ethics Committee or Institutional Review Board for each center.
Men and women 18 years of age or older with at least a 6-mo history of CC were eligible to participate in the study. CC was defined, according to a modification of Rome II criteria, as less than three complete spontaneous bowel movements (CSBMs) per week accompanied by one or more of the following symptoms on more than 25% of occasions: very hard and/or hard stools (type 1 and/or 2 on the Bristol Stool Form Scale[19]), sensation of incomplete evacuation following at least 25% of BMs, and straining on at least 25% of days. All CC-related symptoms were confirmed by patient electronic diary (eDiary) data recorded during the baseline period.
Patients were excluded from entering the study if they had inflammatory bowel disease or other structural bowel disease, CC resulting from bowel surgery (with mechanical outlet obstruction, congenital anorectal malformation or clinically significant rectocele), abdominal pain/discomfort as the most bothersome symptom in the past 6 mo, past or current history or diagnosis of IBS, significant disorders or diseases that may interfere with completion of the study, or if they failed to complete the daily or weekly eDiary assessments during baseline. Patients who planned to use concomitant medications affecting bowel habits (including natural/homeopathic products) 1 wk prior to entry into baseline and during the study were also excluded, as were patients who used laxatives on more than two separate occasions during baseline. However, laxative use (bisacodyl, 15 mg/d) as a rescue medication was allowed for patients who did not experience a BM for at least 72 h.
Patients were excluded from the treatment phase if CC was not confirmed by the baseline eDiary data, if loose or watery stools were reported for 3 or more days during the baseline period, or for lack of compliance with the study protocol.
The primary efficacy variable was the change from baseline in the number of CSBMs per week during the 4-wk treatment period. Secondary efficacy variables included two response rates in terms of CSBMs: patients with a mean increase of one or more CSBM relative to baseline, and patients with an absolute number of three or more CSBMs per week during the 4-wk treatment period. Additional secondary efficacy variables included assessment of patients’ bowel habits [i.e., stool form, frequency, and straining (which was recorded daily regardless of BM)], and patients’ satisfaction with bowel habits, bothersomeness of constipation, distension/bloating, and abdominal discomfort/pain. Following each BM, patients were asked to record the time of the BM, whether the BM was accompanied by a feeling of complete evacuation (yes/no), and stool form (using the Bristol Stool Form Scale[19]). Patients evaluated their satisfaction with bowel habit on a 5-point scale ranging from ‘not at all satisfied’ to ‘a very great deal satisfied’, and bothersomeness of constipation, abdominal distension/bloating and abdominal discomfort/pain on a 5-point scale, which ranged from ‘not at all bothersome’ to ‘a very great deal bothersome’. During the treatment phase of the study, patients also recorded whether they experienced satisfactory relief of constipation symptoms (yes/no) on a weekly basis.
The safety of tegaserod 6 mg b.i.d. vs placebo was evaluated by recording the frequency and severity of all adverse events (AEs) and serious adverse events (SAEs), by monitoring hematology, blood chemistry, urine and vital signs, and by performing electrocardiogram (ECG) evaluations. Other outcomes assessed but not presented in this paper included: patients’ assessment of constipation on quality of life (PAC-QoL questionnaire) and patients’ perception of study medication (PPSM questionnaire).
Planned enrollment was for 600 (n = 300 per arm) randomized patients with CC recruited from 15 centers across China. An assumption was made that the population distribution would be similar to that observed in the two pivotal CC trials[14,15]. Based on this assumption, the study was powered to detect a difference (tegaserod-placebo) in a change from baseline of 0.6 CSBMs/wk at a two-sided significance level of 5%. All statistical analyses were carried out with SAS® software (version 8.2).
As stated earlier, the primary efficacy variable was the change from baseline in the number of CSBMs per week during the 4-wk treatment period. Secondary efficacy variables included two response rates in terms of CSBMs: patients with a mean increase of one or more CSBMs relative to baseline, and patients with an absolute number of three or more CSBMs per week during the 4-wk treatment period. Additional secondary efficacy variables included assessment of patients’ bowel habits [i.e., stool form, frequency, and straining (which was recorded daily regardless of BM)], and patients’ satisfaction with bowel habits, bothersomeness of constipation, distension/bloating, abdominal discomfort/pain, and satisfaction with symptom relief (recorded weekly).
All efficacy analyses were performed on the intent-to-treat (ITT) population. Safety analyses included all patients who received at least one dose of study medication. An analysis of covariance (ANCOVA) model with repeated measures was used to analyze the overall effect of treatment for the primary efficacy variable (change from baseline in number of CSBM per week). The model included terms for treatment, week, study center, and baseline data as well as baseline data by week, and treatment by week interactions. To be defined as ‘complete’, BMs had to be associated with a sensation of complete evacuation. The BM was defined as spontaneous if no laxatives were taken during the 24 h prior to the BM.
Statistical analyses of the secondary efficacy variables [response rate relative to baseline (one or more CSBM per week) and response rate in terms of the absolute number of CSBMs (three or more CSBMs per week)] were carried out using a logistic regression model, with treatment and study center as factors, and the number of CSBMs per week at baseline as covariate.
Based on the daily eDiary assessments, the change from baseline was determined for the following variables: 1) the number of SBMs per week, 2) the number of days per week with no stools, hard or very hard stools, 3) the weekly mean straining score, and 4) the number of days with (too much) straining. The same ANCOVA model that was used for the primary endpoint was repeated for these variables. The number of patients with or without satisfactory relief [determined using a weekly eDiary assessment (patients were asked to consider whether they had satisfactory relief from their symptoms of CC in the past week)] were analyzed using Cochran Mantel Haenszel (CMH) tests, with center as a stratification factor.
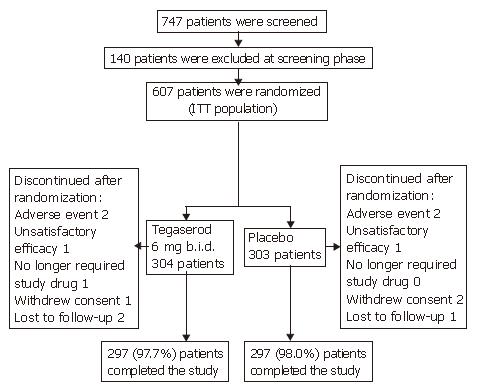
A total of 747 patients were screened for participation in this study, and 607 patients (81.3%) were randomized from a total of 15 centers to receive treatment with tegaserod 6 mg b.i.d. (n = 304) or placebo (n = 303) (Figure 1). The main reasons for screening failure were unacceptable laboratory values (5.0%), withdrawal of consent (4.3%) and ‘other’ (4.3%). Most patients completed the double-blind treatment period (97.7% in the tegaserod group and 98.0% in the placebo group). One patient randomized to receive tegaserod did not receive study medication and was therefore excluded from the safety population.
Demographic and baseline variables were comparable between the tegaserod and placebo groups and most patients were Oriental [99.7% (tegaserod) vs 100.0% (placebo)] (Table 1).
| Demographic variable | Tegaserod 6 mg b.i.d. | Placebo |
| (n = 304) | (n = 303) | |
| Age (yr) | ||
| Median | 34.5 | 35 |
| Range | 18-80 | 18-78 |
| Age group, years (n, %) | ||
| < 35 | 152 (50.0) | 151 (49.8) |
| 35-64 | 132 (43.4) | 135 (44.6) |
| ≥ 65 | 20 (6.6) | 17 (5.6) |
| Sex (n, %) | ||
| Male | 70 (23.0) | 61 (20.1) |
| Female | 234 (77.0) | 242 (79.9) |
| Race (n, %) | ||
| Oriental | 303 (99.7) | 303 (100.0) |
| Other | 1 (0.3) | 0 |
| Mean duration of constipation symptoms, months (SD) | 100.3 (90.7) | 94.9 (93.1) |
Prior to randomization, the duration of patients’ constipation symptoms was approximately 8 years in the tegaserod and placebo groups (Table 1). The mean number of CSBMs per week during the 2-wk baseline period was 0.36 in the tegaserod group and 0.31 in the placebo group. The most bothersome symptoms reported by patients subsequently randomized to tegaserod or placebo was straining (53.0% vs 56.1%), followed by feeling of incomplete evacuation (15.1% vs 15.2%), hard stools (13.5% vs 13.9%) and infrequent defecation (14.5% vs 11.9%). Laxative use in both treatment groups was comparable during the baseline period (35.5% and 36.0% of patients, randomized to tegaserod and placebo, respectively).
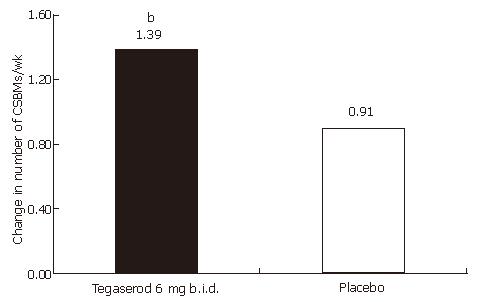
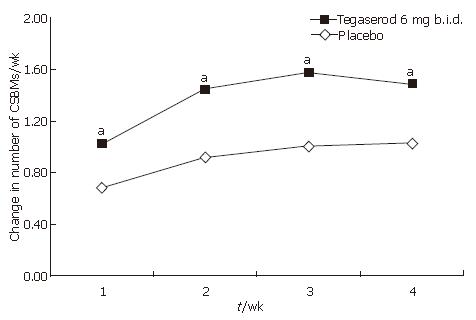
An increase from baseline in the overall number of CSBMs per week during the 4-wk treatment period was observed in patients receiving tegaserod (adjusted mean 1.39) and placebo (adjusted mean 0.91) (treatment difference; 0.48, 95% confidence interval; 0.23-0.73), yielding a statistically significant difference in favor of tegaserod (P = 0.0002, Figure 2 and Table 2). Tegaserod treatment significantly increased the number of CSBMs per week from baseline during each week of treatment, compared with placebo (P < 0.05) (Figure 3 and Table 2). Subgroup analysis revealed that men treated with tegaserod showed a greater increase from baseline in the overall number of CSBMs per week (wk 1-4) compared with women treated with tegaserod (the mean increase in the number of CSBMs per week was 1.67 in men and 1.29 in women).
| Timeperiod | Tegaserod 6mg b.i.d.n = 304 | Placebon = 303 | |
| wk 1-4 | n | 303 | 303 |
| Mean (SD) | 1.38 (1.759) | 0.89 (1.444) | |
| Adjusted mean1 | 1.39 | 0.91 | |
| Median | 0.75 | 0.25 | |
| Min, max | -2.0, 9.0 | -1.5, 7.5 | |
| Tegaserod-placebo | 0.48 | ||
| (95% CI)2 | (0.23, 0.73) | ||
| P value3 | 0.0002 | ||
| wk 1 | Tegaserod–placebo | 0.34 | |
| (95% CI)2 | (0.05, 0.64) | ||
| P value3 | 0.0226 | ||
| wk 2 | Tegaserod-placebo | 0.54 | |
| (95% CI)2 | (0.24, 0.84) | ||
| P value3 | 0.0004 | ||
| wk 3 | Tegaserod–placebo | 0.57 | |
| (95% CI)2 | (0.27, 0.86) | ||
| P value3 | 0.0002 | ||
| wk 4 | Tegaserod–placebo | 0.47 | |
| (95% CI)2 | (0.17, 0.77) | ||
| P value3 | 0.002 | ||
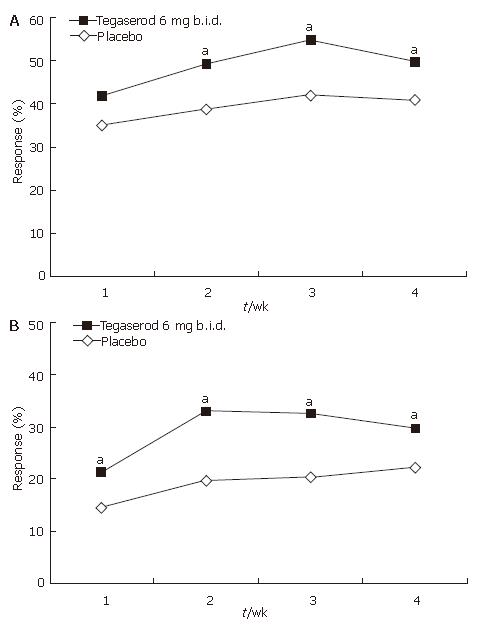
Analysis of response, defined as a mean increase of one or more CSBMs per week relative to baseline during the 4-wk treatment period, showed that treatment with tegaserod was significantly more effective than treatment with placebo [overall, tegaserod (47.7%) vs placebo (35.0%), P = 0.0018). While overall response was statistically significant for all 4 wk combined, statistical significance for individual weeks was reached at wk 2, 3 and 4 of treatment (Figure 4A).
Overall, treatment with tegaserod was superior to treatment with placebo in terms of the absolute response rate (three or more CSBMs per week) [tegaserod (25.0%) vs placebo (14.5%), P = 0.0021]. This difference was statistically significant at each week of treatment (Figure 4B).
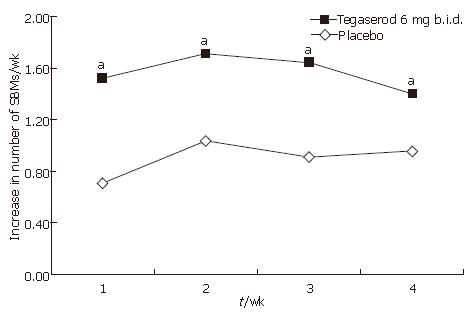
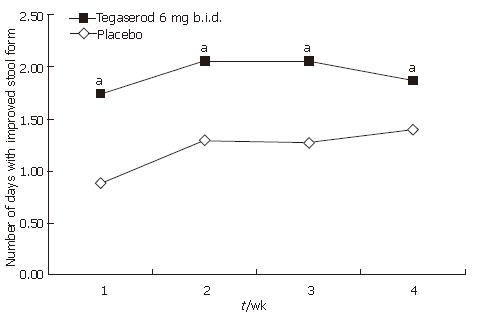
Improvements in constipation symptoms were observed in patients receiving tegaserod over wk 1-4. Tegaserod significantly increased the number of SBMs per week compared with placebo (adjusted mean 1.57 vs 0.89, P < 0.0001), and this was statistically significant for each of the 4 wk of treatment (P < 0.05) (Figure 5). Compared with placebo, treatment with tegaserod also decreased the overall number of days per week (wk 1-4) with no stools, hard or very hard stools (adjusted mean -1.94 vs -1.19, P < 0.0001) and this was statistically significant for each of the 4 wk of treatment (P < 0.05) (Figure 6). Treatment with tegaserod also resulted in a decrease in the weekly mean straining score (adjusted mean -0.41 vs -0.33, P = 0.0282) and a decrease in the number of days per week with straining (adjusted mean -1.65 vs -1.24, P = 0.0085).
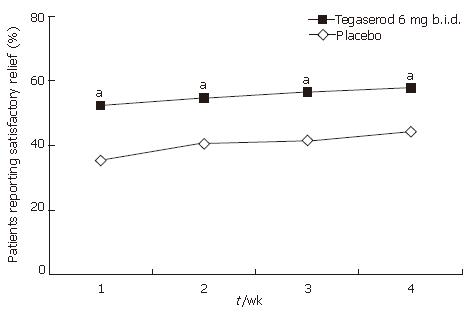
Significantly more patients receiving tegaserod than placebo responded positively to the question of whether they had ‘satisfactory relief from their constipation symptoms over the past week of treatment’ at all 4 wk of treatment (wk 1: 52.5% vs 35.0%; wk 2: 54.9% vs 40.5%; wk 3: 56.5% vs 41.3%; wk 4: 57.8% vs 44.2%; all P < 0.05) and at end of treatment (57.8% vs 43.9, P < 0.05) (Figure 7).
Trends in favor of tegaserod were observed for the following secondary variables: bothersomeness of constipation, distension/bloating and abdominal discomfort/pain. Statistical significance was observed in wk 3 (bothersomeness of distension/bloating and abdominal pain/discomfort) and in wk 1-3 (bothersomeness of constipation). The number of patients using laxatives during the double-blind treatment period was higher in the placebo group (31.4%) compared with the tegaserod group (27.0%), although this difference was not statistically significant.
The AEs reported in this study were mostly mild and transient. The overall frequency of AEs was similar in both the tegaserod and placebo groups (9.9% vs 11.2%) (Table 3). Diarrhea was the most common AE, reported by 3.6% of patients in the tegaserod group and 1.7% of patients in the placebo group. The study investigators considered the majority of cases of diarrhea to be mild (no cases were reported to be severe), and transient [median duration of first episode of diarrhea: 2 d (tegaserod group) vs 3 d (placebo group)]. All cases of abdominal pain were reported to be mild or moderate in severity and were reported with equal frequency in both groups (1.7% in each group).
| Adverse event | Tegaserod 6 mg b.i.d.(n = 303) (%) | Placebo(n = 303) (%) |
| Diarrhea | 11 (3.6) | 5 (1.7) |
| Abdominal pain | 5 (1.7) | 5 (1.7) |
| Nasopharyngitis | 1 (0.3) | 7 (2.3) |
| Transaminases increased | 3 (1.0) | 1 (0.3) |
| Nausea | 1 (0.3) | 3 (1.0) |
| Abdominal distension | 3 (1.0) | 0 |
| Dizziness | 2 (0.7) | 0 |
| Leukopenia | 1 (0.3) | 1 (0.3) |
| Urinary tract inflammation | 1 (0.3) | 1 (0.3) |
| Abdominal pain upper | 0 | 2 (0.7) |
The number of discontinuations due to AEs was the same in both treatment groups (0.7% each) (AEs resulting in discontinuation included diarrhea, dizziness, hypertension, rash, tinnitus, venous thrombosis in the limb, and vertigo). Other reasons for discontinuation included unsatisfactory therapeutic effect (0.3% in each group), patients no longer requiring study drug due to symptom improvement (0.3% in the tegaserod group and 0.0% in the placebo group), withdrawal of consent (0.3% in the tegaserod group and 0.7% in the placebo group) and patients lost to follow-up (0.7% in the tegaserod group and 0.3% in the placebo group). Five SAEs were reported, none of which were considered to be related to the study drug [one case of ureteric cancer in the tegaserod group (0.3%) and one case each of ankle fracture, pregnancy, hemorrhoid surgery, and venous thrombosis in the limb in the placebo group (1.3% in total)]. No cases of ischemic colitis were reported during this study, and no deaths occurred. Laboratory and ECG evaluations, and vital signs were comparable between treatment groups.
This is the first randomized, double-blind, placebo-controlled trial designed to evaluate the efficacy and safety of tegaserod in an adult population of men and women from China, who met the Rome II diagnostic criteria for CC. The recent Rome III criteria, published after this study was conducted, have further refined diagnostic criteria for CC[20].
The key efficacy analyses demonstrated that tegaserod improves multiple symptoms of CC. The results revealed that treatment with tegaserod was associated with a rapid and significant increase from baseline in the number of CSBMs at wk 1, which was sustained over each of the 4 wk of treatment. Secondary efficacy analyses also showed statistically significant improvements for tegaserod over placebo with regard to evaluation of bowel habits and satisfaction with constipation relief.
The responder rate for mean increase of one or more CSBM per week during the 4-wk treatment period [tegaserod (47.7%) vs placebo (35.0%)] was similar to the results obtained with Caucasian patients[14,15] suggesting that patients from China with CC respond in a similar fashion to those from Western countries.
Subgroup analysis confirmed that tegaserod relieved the multiple symptoms of CC in both men and women. This observation has clinical relevance, as fewer data are available in men, and further confirms the results from other pivotal studies[14,15].
Treatment with tegaserod was associated with a safety profile similar to that seen with placebo, although slightly more patients receiving tegaserod than placebo reported mild and transient diarrhea. Diarrhea is a predictable pharmacological event, and is likely due to tegaserod’s promotile effect that stimulates peristalsis, reduces stool hardness and accelerates orocecal transit, promoting stool expulsion[12,13]. The increased incidence of diarrhea following treatment with tegaserod was similar to that reported in all other clinical studies of patients with CC from different ethnic groups[14,15,17,18].
The primary efficacy variable used in this study was the change from baseline in the number of CSBMs per week. The assessment of SBMs discounts laxative-induced BMs, while further characterizing SBMs as ‘complete’ ensures that measurements are not simply based on an increase in the number of small hard pellets that are passed, which would leave symptoms unimproved for the patient. Hence, the definition of CSBM used in this study provides a subjective measure of BMs that are associated with a sense of complete evacuation, while in addition, providing an objective measure of the number of BMs. This assessment of CSBMs is considered to be able to detect changes that are meaningful to the patient.
Medications, such as laxatives, which are traditionally used to treat CC, may improve the frequency of BMs, but they do not treat the underlying causes of CC and have no proven effect on the multiple symptoms including straining, incomplete evacuation, abdominal bloating and abdominal discomfort/pain[9,21]. Therefore, in order to control their symptoms, patients often rely on multiple treatments, which are often ineffective. These include the increased intake of fiber, modifications in lifestyle and diet, and the use of prescription/non-prescription laxatives. This has led to high patient dissatisfaction and frustration with current treatments for CC[1], and hence there is a need for simple, safe, and effective first-line therapies to treat the multiple symptoms of patients with this disorder.
In conclusion, this was the first rigorously designed study to evaluate the efficacy and safety of tegaserod in an adult population of men and women from China with CC. The results of key efficacy analyses demonstrated that compared with placebo, tegaserod 6 mg b.i.d. increased the frequency of CSBMs, improved multiple symptoms of constipation, and was associated with a safety profile that is similar to that of placebo. Therefore, tegaserod offers an effective treatment option for patients from China with CC.
The study design, collection, analysis and interpretation of the data was sponsored by Novartis Pharma AG. The authors would like to acknowledge the editorial support and contribution of Nicci Crofts of ACUMED® to this manuscript. ACUMED’s contribution was funded by Novartis Pharma AG.
Chronic constipation/idiopathic constipation (CC) affects 11% to 14% of the Chinese population; however, currently prescribed first-line therapies for CC are suboptimal. Tegaserod is a selective partial agonist at the serotonin receptor, 5-HT4, which is a well tolerated and effective treatment for the multiple symptoms of CC in Caucasian patients. However, its efficacy in patients from Asia-Pacific countries is unknown.
There has been increasing interest in the use of serotonergic agents, such as tegaserod, for the treatment of gastrointestinal motility disorders. This study is the first randomized, double-blind, placebo-controlled trial designed to evaluate the efficacy and safety of tegaserod in men and women from China with CC.
The placebo-controlled trials demonstrating the promotile action of tegaserod on the gut (Degen et al, 2000 and Prather et al, 2000) led to the design of phase III trials of tegaserod in patients with irritable bowel syndrome whose predominant symptom was constipation (IBS-C) and patients with CC. These studies demonstrated that treatment with tegaserod relieved multiple symptoms of these burdensome conditions (Johanson et al, 2004; Kamm et al, 2005; Müller-Lissner et al, 2001; Novick et al, 2002; Tack et al, 2005). As these studies were performed predominantly in patients from Western countries, this study aimed to evaluate the efficacy of tegaserod for the treatment of CC in patients from China.
The results of this study demonstrated that compared with placebo, tegaserod 6 mg b.i.d. increased the frequency of CSBMs, improved multiple symptoms of constipation, and was well tolerated in both men and women with CC from China.
Complete spontaneous bowel movement (CSBM): The term ‘CSBM’ refers to the spontaneous occurrence of a bowel movement associated with a feeling of complete evacuation. Assessing bowel movements as ‘spontaneous’ discounts laxative-induced bowel movements, but further characterizing spontaneous bowel movements as ‘complete’ (CSBMs) ensures that measurements are not simply based on an increase in the number of small hard pellets that are passed, which would leave symptoms unimproved for the patient. The definition of CSBM used in this study therefore provides a subjective measure of bowel movements that are associated with a sense of complete evacuation, while in addition, providing an objective measure of the number of bowel movements. Tegaserod: A selective partial agonist of the 5-HT4 receptor.
This is an excellently designed, performed and presented study examining the efficacy of tegaserod in Chinese patients with chronic idiopathic constipation.
S- Editor Liu Y L- Editor Lutze M E- Editor Lu W
| 1. | Cheng C, Chan AO, Hui WM, Lam SK. Coping strategies, illness perception, anxiety and depression of patients with idiopathic constipation: a population-based study. Aliment Pharmacol Ther. 2003;18:319-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Brandt LJ, Prather CM, Quigley EM, Schiller LR, Schoenfeld P, Talley NJ. Systematic review on the management of chronic constipation in North America. Am J Gastroenterol. 2005;100 Suppl 1:S5-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Garrigues V, Gálvez C, Ortiz V, Ponce M, Nos P, Ponce J. Prevalence of constipation: agreement among several criteria and evaluation of the diagnostic accuracy of qualifying symptoms and self-reported definition in a population-based survey in Spain. Am J Epidemiol. 2004;159:520-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Guo XK, Pan MY, Han G, Fang S, Lu XC, Guo H. A cluster, stratified, randomized epidemiologic survey and analysis of related factors on adult chronic constipation in Beijing area. Chin J Dig. 2002;22:637-638. [Cited in This Article: ] |
| 5. | Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360-1368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 495] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Norton C. Constipation in older patients: effects on quality of life. Br J Nurs. 2006;15:188-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Wald A. Pathophysiology, diagnosis and current management of chronic constipation. Nat Clin Pract Gastroenterol Hepatol. 2006;3:90-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead W. Rome II: The Functional Gastrointestinal Disorders. 2nd ed. McLean, VA: Degnon Associates 2000; . [Cited in This Article: ] |
| 9. | Xing JH, Soffer EE. Adverse effects of laxatives. Dis Colon Rectum. 2001;44:1201-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Schiller LR. New and emerging treatment options for chronic constipation. Rev Gastroenterol Disord. 2004;4 Suppl 2:S43-S51. [PubMed] [Cited in This Article: ] |
| 12. | Degen L, Matzinger D, Merz M, Appel-Dingemanse S, Maecke H, Beglinger C. Tegaserod (HTF 919), a 5-HT4 receptor partial agonist, accelerates gastrointestinal (GI) tract. Neurogastroenterol Motil. 2000;12:382. [Cited in This Article: ] |
| 13. | Prather CM, Camilleri M, Zinsmeister AR, McKinzie S, Thomforde G. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 318] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Johanson JF, Wald A, Tougas G, Chey WD, Novick JS, Lembo AJ, Fordham F, Guella M, Nault B. Effect of tegaserod in chronic constipation: a randomized, double-blind, controlled trial. Clin Gastroenterol Hepatol. 2004;2:796-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Kamm MA, Müller-Lissner S, Talley NJ, Tack J, Boeckxstaens G, Minushkin ON, Kalinin A, Dzieniszewski J, Haeck P, Fordham F, Hugot-Cournez S, Nault B. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Müller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E, Nault B, Rüegg P. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 17. | Novick J, Miner P, Krause R, Glebas K, Bliesath H, Ligozio G, Rüegg P, Lefkowitz M. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16:1877-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 221] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Tack J, Müller-Lissner S, Bytzer P, Corinaldesi R, Chang L, Viegas A, Schnekenbuehl S, Dunger-Baldauf C, Rueegg P. A randomised controlled trial assessing the efficacy and safety of repeated tegaserod therapy in women with irritable bowel syndrome with constipation. Gut. 2005;54:1707-1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990;300:439-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 352] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3413] [Cited by in F6Publishing: 3270] [Article Influence: 181.7] [Reference Citation Analysis (1)] |
| 21. | Müller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 260] [Article Influence: 13.7] [Reference Citation Analysis (1)] |