Published online Dec 28, 2007. doi: 10.3748/wjg.v13.i48.6512
Revised: August 27, 2007
Accepted: August 30, 2007
Published online: December 28, 2007
AIM: To investigate the anti-neoplastic effects of MK615, an extract from the Japanese apricot (Prunus mume), against colon cancer cells.
METHODS: Three colon cancer cell lines, SW480, COLO, and WiDr, were cultured with MK615. Growth inhibition was evaluated by cell proliferation assay and killing activity was determined by lactate dehydrogenase assay. Induction of apoptosis was evaluated by annexin V flow cytometry. Morphological changes were studied by light and electron microscopy, and immunofluorescence staining with Atg8.
RESULTS: MK615 inhibited growth and lysed SW480, COLO and WiDr cells in a dose-dependent manner. Annexin V flow cytometry showed that MK615 induced apoptosis after 6 h incubation, at which point the occurrence of apoptotic cells was 68.0%, 65.7% and 64.7% for SW480, COLO, and WiDr cells, respectively. Light and electron microscopy, and immunofluorescence staining with Atg8 revealed that MK615 induced massive cytoplasmic vacuoles (autophagosomes) in all three cell lines.
CONCLUSION: MK615 has an anti-neoplastic effect against colon cancer cells. The effect may be exerted by induction of apoptosis and autophagy.
-
Citation: Mori S, Sawada T, Okada T, Ohsawa T, Adachi M, Keiichi K. New anti-proliferative agent, MK615, from Japanese apricot “
Prunus mume ” induces striking autophagy in colon cancer cellsin vitro . World J Gastroenterol 2007; 13(48): 6512-6517 - URL: https://www.wjgnet.com/1007-9327/full/v13/i48/6512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i48.6512
Japanese apricot, Prunus mume Sieb. et Zucc. (known as ume in Japanese), has for centuries been a traditional Japanese medicine, and is a familiar and commonly consumed food. Prunus mume is a species of Asian plum of the family Rosaceae. It is often called a plum, but it is more closely related to the apricot (ume in Japanese). MK615, an extract of compounds from Japanese apricot, has been shown to possess an anti-proliferative effect against some cancer cell lines[1,2]. Although MK615 induces apoptosis of breast cancer cells and some other human cancer cells, morphological studies have revealed the appearance of abundant cytoplasmic vacuoles early after MK615 exposure, which are not relevant to apoptosis[2]. The precise role of these vacuoles has not been elucidated.
Even though effective chemotherapeutic agents and regimens have been developed, colorectal cancer is still associated with high rates of morbidity and mortality worldwide[3,4]. Furthermore, the side effects of chemotherapeutic agents often hamper the quality of life of patients with colorectal cancer. There is a need, therefore, to develop new, effective and less toxic chemotherapeutic agents against colorectal cancer.
In the present study, we investigated the antineoplastic effects of MK615 against colon cancer cell lines in vitro. We found that MK615 strongly induced autophagy in colon cancer cells and exerted antineoplastic effects.
MK615, derived from the fruit of the Japanese apricot[1], was provided by the Japan Apricot Co. (Gunma, Japan). The preparation involved the extraction of apricot juice using a press. This was then heated and concentrated. The concentrate was dissolved in diethylether, which was then completely removed from the extract by rotary evaporation. The dried hydrophobic extract, MK615, was dissolved in dimethyl sulfoxide (DMSO; Wako Pure Chemical Industry, Osaka, Japan) at several concentrations. The MK615/DMSO solution was applied to cell cultures at a concentration of 10 μL/mL of culture medium. DMSO alone was added as a negative control.
Three human colon cancer cell lines, SW480, COLO, and WiDr, were purchased from the American Type Culture Collection (Manassas, VA, USA). These three were selected because of the differences in sex, race, and the productivity of proteins, such as carcinoembryonic antigen (CEA). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal calf serum (FCS) in a 5% CO2 incubator.
The cells were plated at 1 × 104/well in 96-well plates in DMEM containing 10% (v/v) FCS. The cells were then cultured with or without MK615 at concentrations of 150, 300 or 600 μg/mL. For negative control wells, cells were cultured with 1% DMSO alone. After 48 h, 15 μL MTT (3-(4,5-dimethylthiazol-2-yl)-5-(3carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt) (5 g/L) was added to each well, and the cells were incubated for 4 h. Then, 100 μL solubilization solution/stop mix was added following the manufacturer’s instructions (Promega, Madison, WI, USA), and the plates were left to stand for 60 min. Absorbances at 570 nm and 630 nm were measured with an ELISA reader. Actual counts were calculated by subtracting the absorbance at 570 nm from that at 630 nm. Each assay was performed in triplicate and the average absorbance was calculated. The data presented here were calculated using the ratio of absorbance at each drug concentration to the absorbance in the absence of drugs.
LDH assay was performed using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). Briefly, cells were plated at 5 × 103/well in 96-well plates in DMEM containing 10% (v/v) FCS, then cultured with or without MK615 at 150, 300 or 600 μg/mL. For the negative control wells, cells were cultured with 1% DMSO alone. The medium was removed and 100 μL CytoTox-ONE Reagent was added. Cells were then incubated at 22°C for 10 min and 50 μL stop solution added. The plates were shaken for 10 s and the absorbances at 560 nm and 590 nm measured with an ELISA reader. To obtain the maximum LDH release, 10 μL lysis solution (10 ×) was added to the positive control wells 45 min prior to harvest. After 48 h, a 50 μL supernatant was transferred to a fresh 96-well plate, and incubated for 30 min with 50 μL substrate mix at room temperature. Then, 50 μL stop solution was added, and the absorbance at 490 nm measured with an ELISA reader. Each assay was performed in triplicate and the average absorbance calculated. The data presented here were calculated using the formula: percentage cytotoxicity = 100 × (experimental - culture medium background) /(maximum LDH release - culture medium and lysis solution background).
Cells were cultured with MK615 at a concentration of 300 μg/mL for 6 h, then harvested by trypsinization and washed twice with PBS. Annexin V binding assay was performed using the Annexin V-FITC Apoptosis Detection kit (Becton-Dickinson, NJ) according to the supplier’s instructions. At least 1 × 106 cells were incubated with FITC-conjugated annexin V at room temperature for 15 min. Cells were then analyzed on a FACScalibur flowcytometer (Becton-Dickinson).
Cells were cultured with MK615 at a concentration of 300 μg/mL for 6 h, then collected by trypsinization. Collected cells were mounted on glass slides by the spin-down procedure (50 rpm, 3 min) and stained with Diff Quick staining solution (International Reagents, Kobe, Japan). Morphological changes were assessed by light microscopy of Diff Quick specimens.
Cells were cultured with MK615 at concentrations of 150, 300 or 600 μg/mL for 6 h, then fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4), followed by 1% osmium tetroxide. After dehydration, thin sections were stained with uranyl acetate and lead citrate for observation using a JEM 100 CX electron microscope (JEOL, Peabody, NY, USA).
Cells were cultured with MK615 at a concentration of
300 μg/mL for 6 h, then fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. They were then washed three times in PBS containing 0.01% Triton X-100 and 10% FBS, followed by incubation with anti-autophagy cleaved-APG8b (MAPILC3B) antibody (ABGENT, CA, USA), or 4’, 6-diamino-2-phenylindole (DAPI) nucleic acid stain (Lonza, Basel, Switzerland).
Statistical analysis for the cell proliferation assay and LDH assay was performed by Student’s t test, comparing the counts at 0 μg/mL MK615 with those at each concentration of MK615. The percentage inhibition was calculated using the ratio of absorbance at each concentration of MK615 relative to the absorbance with no drug added.
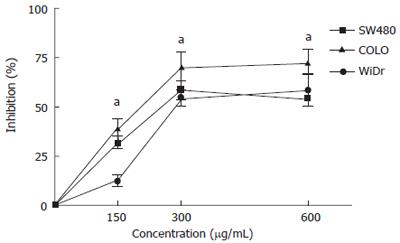
The anti-proliferative effects of MK615 against colon cancer cells were evaluated by cell proliferation assay (Figure 1). The percentage inhibition rates of SW480 at 150, 300 and 600 μg/mL MK615 were 31.9%, 58.5% and 54.2%, respectively, and those of COLO and WiDr were 38.9%, 70.4% and 72.4%, and 12.1%, 54.5% and 58.3%, respectively. The inhibition induced by MK615 at all concentrations was significantly higher than that in the absence of MK615, and was dose-dependent in the COLO and WiDr cell lines.
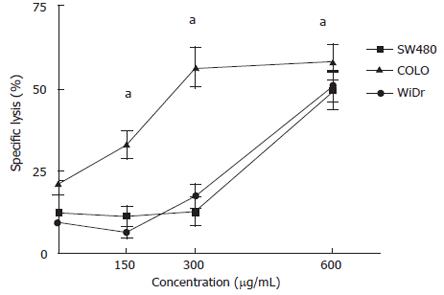
We examined the ability of MK615 to kill colon cancer cells using a LDH-releasing assay (Figure 2). The percentage specific lysis of SW480 at 0, 150, 300 and 600 μg/mL MK615 was 12.5%, 11.3%, 12.8% and 49.6%, respectively. The respective values for COLO and WiDr were 21.4%, 33.1%, 56.6 and 58.3%, and 9.4%, 6.4%, 17.4% and 50.7%. The percentage specific lysis at all concentrations was significantly higher than that for the controls in all three cell lines.
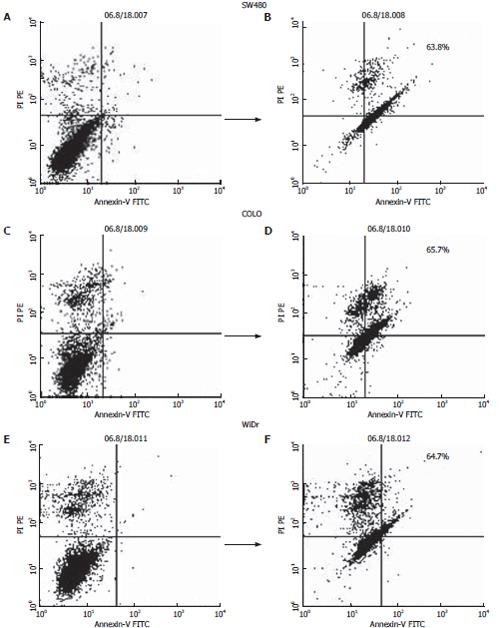
The antiproliferative effect of MK615 is partly attributed to the induction of apoptosis[2]. As shown in Figure 3, MK615 treatment induced apoptosis in all three colon cancer cell lines. After incubation with 300 μg/mL MK615 for 6 h, the frequencies of apoptotic cells in SW480, COLO and WiDr cells were 68.0%, 65.7% and 64.7%, respectively.
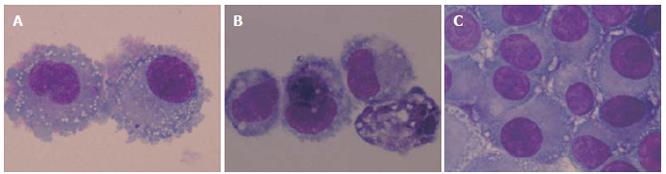
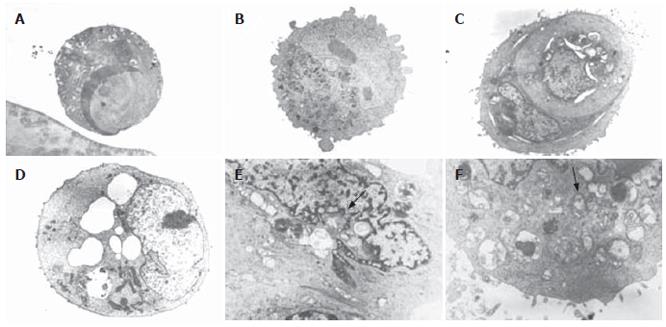
It has also been reported that MK615 treatment induces the formation of cytoplasmic vacuoles in breast cancer cells[2]. Similarly, in the present study with colon cancer cells, abundant cytoplasmic vacuoles were observed in all three cell lines after 6 h incubation with MK615 at 300 μg/mL (Figure 4). Electron microscopy revealed that the cytoplasmic vacuoles were typical autophagosomes. Although some cells showed typical features of apoptosis (Figure 5A), there were abundant autophagosomes showing a membrane structure within which cytoplasmic structures were entrapped (Figure 5B-5F). In some cells, degeneration of mitochondria was observed (Figure 5).
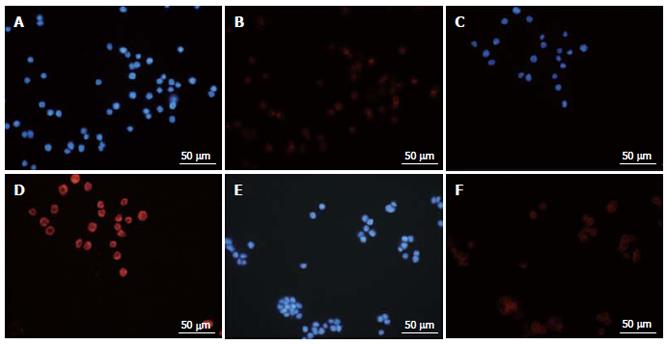
Immunofluorescence staining with Atg8 (LC3) showed positive labeling in all three cell lines after treatment with MK615 (Figure 6). Nuclei were stained blue with DAPI (Figure 6A, 6C and 6E), and Atg8 (LC3) produced peripheral red staining (Figure 6B, 6D and 6F).
In the present study, we showed that MK615 inhibited the growth of three colon cancer cell lines, SW480, COLO, and WiDr, and induced massive autophagy. Apoptosis is a well known form of programmed cell death (PCD), and is widely accepted as the main mechanism of cancer cell death[5-8]. Currently, apoptosis is classified as a typeIPCD, whereas autophagy is classified as type II PCD[9-13]. Autophagy differs in several ways from typeIPCD. Autophagy is a form of caspase-independent cell death, displays no DNA-laddering, and is typically characterized by the formation of cytoplasmic vacuoles. In recent years, the importance of autophagy has been stressed in various biological fields, including cancer[14-16]. Autophagy is an evolutionarily conserved pathway that delivers and recycles cytoplasmic components, such as mitochondria and Golgi apparatus. Thus, starvation is a typical trigger that can induce autophagy. Some studies have indicated that cancer cells show less autophagy than normal cells[17,18], which suggests that induction of autophagy is an attractive mode of anti-cancer therapy.
Autophagy can be induced by various agents, such as tamoxifen[19], γ-irradiation[20,21], and rapamycin[22,23], but few studies have investigated the induction of autophagy by natural substances. A previous study has found that soybean B-group triterpenoid saponins induce macroautophagy in human colon cancer cells[24].
Atg8 is a 117-amino-acid protein essential for the early phase of autophagy[25]. LC3 is a mammalian homologue of Atg8 and is the only established marker of autophagy in mammalian cells[26]. Conditions such as nutrient starvation initiate the formation of a double-membrane structure, which elongates to form a cytoplasmic vesicle, the autophagosome. Finally, autophagosomes fused with lysosomes generate single-membrane autophagolysosomes, resulting in the degradation of their content. Atg8 (LC3) binds to the membrane of the autophagosome and plays a crucial role in the process of autophagy[27,28]. In the present study, MK615 induced the expression of Atg8 (LC3) in all the colon cancer cell lines within 6 h of incubation. Expression of Atg8 (LC3) was exclusively localized in the cytoplasm. These findings indicated that MK615 induced massive autophagy in colon cancer cells in vitro.
Precise elucidation of the mechanism responsible for the antineoplastic effect of MK615 will require further study, as it remains unclear whether autophagy suppresses tumorigenesis or provides cancer cells with a rescue mechanism under unfavorable conditions. Kondo et al have reported that manipulation of autophagy may have different effects in different types of cancer cells. If cancer cells show defective autophagy, induction of autophagy may lead to the inhibition of cancer growth, whereas if cancer cells show protective autophagy in response to chemotherapy, this may decrease their sensitivity to anti-cancer treatment[29].
Importantly, Boya et al have reported that when cells are inhibited at the final step of autophagy, which is the fusion of autophagosome and lysosome, cells die during typeIPCD (apoptosis); on the other hand, when cells complete the whole process of autophagy, they survive. Thus, the inhibition of autophagy causes cells to undergo apoptosis, which suggests that there is cross-talk between typeIand type II PCD[30]. Indeed, the results of annexin-V flow cytometry indicated that MK615 induced apoptosis after 6 h of incubation.
In conclusion, we demonstrated that an extract of compounds from Japanese apricot (ume), MK615, exhibits anti-proliferative effects against colon cancer cells in vitro, and induced massive autophagy in these cells. Although further study is needed, the natural compounds in MK615 appear to exert antineoplastic effects through induction of autophagy-related PCD in colon cancer cells.
The authors thank Dr. Keiichiro Yamaguchi for his excellent assistance with electron microscopy.
Japanese apricot, Prunus mume Sieb. et Zucc (ume in Japanese), has for centuries been a traditional Japanese medicine, and is a familiar and commonly consumed food. Some components of Japanese apricot have been shown to inhibit cancer cell growth.
MK615 is an extract of compounds from Japanese apricot, and has been shown to possess an anti-proliferative effect against some cancer cell lines. Autophagy is a form of PCD and has been shown to regulate cancer-cell growth. However, there are not many substances that can stably induce autophagy in cancer cells.
In this report, we clearly described that MK615 inhibited the growth of colon cancer cells by aggressively inducing autophagy.
The side effects of chemotherapeutic agents often reduce the quality of life of patients with colorectal cancer. MK615 appears to be an effective and less toxic chemotherapeutic agent against colorectal cancer.
Autophagy is classified as type II PCD, and differs from typeIPCD (apoptosis). Autophagy is a form of caspase-independent cell death, displays no DNA-laddering, and is typically characterized by formation of cytoplasmic vacuoles. In recent years, the importance of autophagy has been stressed in various biological fields, including cancer.
In this in vitro study, the authors investigated the antineoplastic effects of MK615, an extract from Japanese apricot (ume in Japanese), against colon cancer cells. They concluded that MK615 had an antineoplastic effect against colon cancer cells, and the effect may have been exerted by induction of apoptosis and autophagy.
S- Editor Zhu LH L- Editor Kerr C E- Editor Wang HF
| 1. | Adachi M, Suzuki Y, Mizuta T, Osawa T, Adachi T, Osaka K, Suzuki K, Shiojima K, Arai Y, Masuda K. The Japanese apricot Prunus mume Sieb. et Zucc (Ume) is a rich natural source of novel anti-cancer substance. Int J Food Prop. 2007;10:375-384. [Cited in This Article: ] |
| 2. | Nakagawa A, Sawada T, Okada T, Ohsawa T, Adachi M, Kubota K. New antineoplastic agent, MK615, from UME (a Variety of) Japanese apricot inhibits growth of breast cancer cells in vitro. Breast J. 2007;13:44-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Terstriep S, Grothey A. First- and second-line therapy of metastatic colorectal cancer. Expert Rev Anticancer Ther. 2006;6:921-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Monga DK, O'Connell MJ. Surgical adjuvant therapy for colorectal cancer: current approaches and future directions. Ann Surg Oncol. 2006;13:1021-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Penn LZ. Apoptosis modulators as cancer therapeutics. Curr Opin Investig Drugs. 2001;2:684-692. [PubMed] [Cited in This Article: ] |
| 6. | Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 416] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 7. | Kabore AF, Johnston JB, Gibson SB. Changes in the apoptotic and survival signaling in cancer cells and their potential therapeutic implications. Curr Cancer Drug Targets. 2004;4:147-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Meiler J, Schuler M. Therapeutic targeting of apoptotic pathways in cancer. Curr Drug Targets. 2006;7:1361-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Bursch W, Ellinger A, Gerner C, Fröhwein U, Schulte-Hermann R. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others. Ann N Y Acad Sci. 2000;926:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Bursch W, Hochegger K, Torok L, Marian B, Ellinger A, Hermann RS. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J Cell Sci. 2000;113:1189-1198. [PubMed] [Cited in This Article: ] |
| 11. | Huang WP, Klionsky DJ. Autophagy in yeast: a review of the molecular machinery. Cell Struct Funct. 2002;27:409-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med. 2003;9:65-76. [PubMed] [Cited in This Article: ] |
| 13. | Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542-1552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 1111] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 14. | Corcelle E, Nebout M, Bekri S, Gauthier N, Hofman P, Poujeol P, Fénichel P, Mograbi B. Disruption of autophagy at the maturation step by the carcinogen lindane is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity. Cancer Res. 2006;66:6861-6870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Ogier-Denis E, Codogno P. Autophagy: a barrier or an adaptive response to cancer. Biochim Biophys Acta. 2003;1603:113-128. [PubMed] [Cited in This Article: ] |
| 16. | Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891-2906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1027] [Cited by in F6Publishing: 1053] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 17. | Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol. 2004;2:301-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 343] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 18. | Otsuka H, Moskowitz M. Differences in the rates of protein degradation in untrasformed and transformed cell lines. Exp Cell Res. 1978;112:127-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Bursch W, Ellinger A, Kienzl H, Török L, Pandey S, Sikorska M, Walker R, Hermann RS. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996;17:1595-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 390] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Yao KC, Komata T, Kondo Y, Kanzawa T, Kondo S, Germano IM. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy. J Neurosurg. 2003;98:378-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Gewirtz DA. Autophagy as a mechanism of radiation sensitization in breast tumor cells. Autophagy. 2007;3:249-250. [PubMed] [Cited in This Article: ] |
| 22. | Takeuchi H, Kanzawa T, Kondo Y, Komata T, Hirohata S, Kyo S, Kondo S. Combination of caspase transfer using the human telomerase reverse transcriptase promoter and conventional therapies for malignant glioma cells. Int J Oncol. 2004;25:57-63. [PubMed] [Cited in This Article: ] |
| 23. | Kim KW, Mutter RW, Cao C, Albert JM, Freeman M, Hallahan DE, Lu B. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 2006;281:36883-36890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Ellington AA, Berhow M, Singletary KW. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis. 2005;26:159-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151:263-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 736] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 26. | Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 401] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 27. | Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37:771-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 340] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 28. | Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 812] [Cited by in F6Publishing: 871] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 29. | Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1283] [Cited by in F6Publishing: 1319] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 30. | Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1271] [Cited by in F6Publishing: 1288] [Article Influence: 67.8] [Reference Citation Analysis (0)] |