Published online Jun 28, 2007. doi: 10.3748/wjg.v13.i24.3354
Revised: January 20, 2007
Accepted: January 25, 2007
Published online: June 28, 2007
AIM: To study the loss of heterozygosity (LOH) at 8p21-23 locus in diffuse gastric cancer.
METHODS: To evaluate the involvement of this region in gastric cancer, we used eight microsatellite markers covering two Mb of mentioned region, to perform a high-resolution analysis of allele loss in 42 cases of late diffuse gastric adenocarcinoma.
RESULTS: Six of these STS makers: D8S1149, D8S1645, D8S1643, D8S1508, D8S1591, and D8S1145 showed 36%, 28%, 37%, 41%, 44% and 53% LOH, respectively.
CONCLUSION: A critical region of loss, close to the NAT2 locus and relatively far from FEZ1 gene currently postulated as tumor suppressor gene in this region.
- Citation: Hosseini HA, Ahani A, Galehdari H, Froughmand AM, Hosseini M, Masjedizadeh A, Zali MR. Frequent loss of heterozygosity at 8p22 chromosomal region in diffuse type of gastric cancer. World J Gastroenterol 2007; 13(24): 3354-3358
- URL: https://www.wjgnet.com/1007-9327/full/v13/i24/3354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i24.3354
Adenocarcinoma of the stomach (ACS) is the second most common cancer worldwide and there are two distinct biological and etiological subtypes of ACS: (a) the intestinal and (b) the diffuse-infiltrative type. The individual’s risk of the intestinal disease is dominant in countries with a high incident of gastric cancer. Although its incidence is decreasing, ACS is the second leading cause of cancer mortality in many countries[1,2].
A recent cancer survey by the Iranian Ministry of Health and Medical Education (IMHME) revealed that gastric adenocarcinoma is the most common fatal cancer in Iran, with a wide variation of death rate among different provinces. According to the recent cancer statistics, deaths due to gastric cancer constitute about 39% of all deaths due to cancer each year in some parts of Iran[3].
Diet and environment are important factors in the intestinal form of ACS, which is associated with chronic atrophic gastritis and intestinal metaplasia of the gastric mucosa. In addition, environmental factors have influence on incidence of the diffuse (i.e. infiltrative) form of ACS[4].One of the strong tools to genetic analyses is loss of heterozygosity (LOH) that consequently leads to loss of function of tumor suppressor genes. Inactivation of first normal allele mainly occurs by point mutation, followed by deletion or loss of second allele[5]. Hence, to LOH analyzing in a region, usually the microsatellite STS markers are used. These markers enable to trail contemporary two alleles of a gene[6].
Frequent LOH at specific chromosomal regions in certain tumors implies the presence of suppressor genes. Recent allelotyping studies have shown that allelic losses on the short arm of chromosome 8, particularly at bands 21-23.1, are frequently associated with various tumors, including prostate cancer[7,8], breast cancer[9,10], head and neck squamous cell carcinoma[11,12], urinary bladder carcinoma[13,14], hepatocellular carcinoma[15], lung cancer[16] and colorectal cancer[17]. Additionally, frequent deletion at 8p22 has been shown strongly associated with gastric cancer progression[18]. These observations suggest that chromosomal region 8p21-23.1 plays a critical role in the development of various tumors.
Experimentally functional evidence by chromosome transfer into tumor cells at 8p region showed the presence of one or more putative tumor suppressor gene(s) in this region[19]. In addition, micro-cell fusion experiments suggested the possible location of metastasis suppressor gene(s) at 8p[20,21]. It is possible that at the locus 8p, two or more genes will be involved in suppressing of cancer development. Efforts toward positional cloning of the suppressor gene(s) allowed the isolation of an important candidate tumor suppressor FEZ1 gene at 8p22. However, the significance of FEZ1 in the tumor-development or progression remains confused[22-24].
Accordingly, in this first study from Iran, eight microsatellite STS markers were selected to analyze frequency of allelic loss in 42 cases of late diffuse type of gastric cancer.
Paraffin embedded tissues of 42 patients were analyzed with advanced locally diffuse type of gastric cancer registered in RCGLD registry system from 2003 to 2005.
DNA Extraction: Formalin-fixed, paraffin-embedded tissue blocks were sectioned with 5 μm thickness. They stained (Hematoxylin and Eosin), and viewed to confirm histological grading. Using the stained dissected slides as templates, two 20-μm section fragment of paraffin-embedded tissue were placed in two sterile tubes as source of tumoral and normal samples, separately. Deparaffinization was performed with Xylene, followed by DNA extraction as described previously[25].
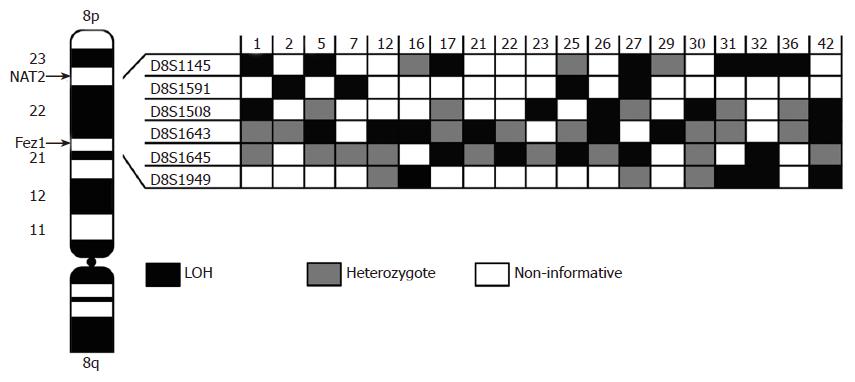
LOH analysis by paired normal-tumor microsatellite PCR was performed using eight well-mapped microsatellite markers (Table 1). These markers cover approximately two Mb of 8p22 region. Figure 1 shows the chromosomal positions of the selected markers, based on the sequence tagged site database (http://www.ncbi.nlm.nih.gov), with supplementary mapping information, provided through the Cooperative Human Linkage Center database (http://www.chlc.org), the Genome Database (http://www.gdb.org), the Genetic Location Database (http://www.cedar.genetics.soton.ac.uk/public_html).
| Locus | Forward primer (5'-3') | Reverse primer (5'-3') | Size range (bp) | Annealingtemperature (°C) | (%) Informative | (%) LOH |
| D8S1948 | TTACAAAACATACCCAGTGTTTGG | CTTTTTAGTGCTTGAGACTGTCTCC | 110-111 | 58 | < 10 | Exclude |
| D8S1949 | TGTCTTACAGCTCTCCCTCTCC | CAGTAAGGATCACCAAGACAAGG | 106-107 | 65 | 40 | 36 |
| D8S1645 | GTTCAACTGTTGATTTTTTGACAA | CTTTTTAGTTAATTCCCATCAGCA | 176-177 | 62 | 72 | 28 |
| D8S1643 | AGGCCTTGTAAGTGATAAAGGC | TTCTCCATCAACCTTTTGGC | 100-101 | 64 | 80 | 37 |
| D8S280 | CAATTTCATTGCTAGGTGTATATCC | CTGTTTTATGGCTGAATAGTGTCC | 224-232 | 59.5 | < 10 | Exclude |
| D8S1508 | AAAATTCCTACCTTGCTATGAACA | CTGCACGTAACTCTCCACCA | 181-182 | 61.5 | 50 | 41 |
| D8S1591 | CAAAGATTTCTTTTTATTCACCTGC | TTTCTTTAGATGGAGTCCATTGC | 208-209 | 59.5 | 36 | 44 |
| D8S1145 | TGCTAACTGGCACGGTCAC | CAATCCCAGTAATCTATAACTTCA | 261-289 | 63 | 56 | 53 |
In a total volume of 25 μL the PCR contained 2 μL DNA sample solution, 200 μm of all four deoxynucleotide triphosphates (dNTP), 50 pmol of each forward and reverse primers, 0.2 μL super Taq polymerase (Roche), 2 μL of 10X PCR buffer (Roche), and 3 mmol/L MgCl2 (Roche). The following thermal cycling conditions were employed for all reactions: an initial denaturation step of 5 min, followed by 35 cycles of denaturing, annealing, and extension (30 s each) and a final 20-min extension step. A denaturing temperature of 95°C and an extension temperature of 72°C were used and annealing temperatures for the different primer sets were optimized as necessary (Table 1)
PCR-products were size-separated on a Biorad 165-3860 Sequi-gene using 5 μL of the sample was then loaded onto a 60 g/L polyacrylamide gel containing 7 mol/L urea, 450 mmol/L Tris-borate (pH 7.5) and 1 mmol/L EDTA (pH 7.0) running buffer. Loaded gels were electrophoresed for 2-4 h (depend on PCR product size bands) and stained with AGNO3 method[26].
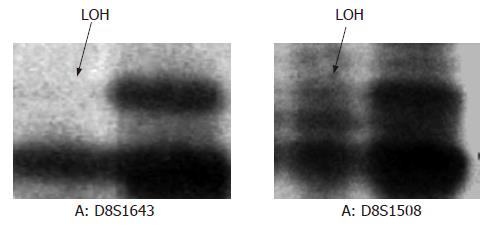
Because of low quality of specimens, 17 of 42 samples were excluded and the remains normal and tumor paired-samples from 25 late diffuse type of gastric cancer were screened for LOH at 8p22. LOH analysis was performed as described previously[27,28]. Each locus scored for LOH according to the absence (allelic loss) or the disequilibrium (allelic imbalance) of signal from one allele in the tumor-DNA-amplification product as compared with the normal one. Reduction of > 50% of band intensity was considered to loss. (Figure 2)
Allelic loss for at least one locus detected in 76% (19/25) cases examined. Eight loci were tested in all of the matched normal and tumor samples (Table 1). Two of these STS markers (D8S1948, D8S280) have been excluded because of non-informatively. In other markers allelic loss ranged were 36% (4/11) for D8S1949, 28% (5/18) for D8S1645, 37% (7/19) for D8S1643, 41% (5/12) for D8S1508, 44% (4/9) for D8S1591 and 53% (7/13) for D8S1145. Allelic imbalance for at least one locus found in 76% of tumor samples. Figure 2 shows the pattern of allelic loss for each case. In eight cases, loss of one allele tends to telomeric and in four cases to centromeric region. Other cases showed loss in the middle point of 8p22 region.
In this study we examined a region of chromosome 8p favored as potentially harboring tumor suppressor genes[7-18]. The allelotyping performed in a region less than two Mb at 8p22 to identify a common deletion in the late diffuse type of gastric carcinoma. Allelic imbalance for at least one locus found in 76% of tumor samples. However, the diffuse-type of gastric cancer has a higher normal cell content and the DNA impurities maybe superimposed to infrequent chromosomal losses[28]. Therefore, the frequency of 8p22 deletion in this study is considerable. The unique connection of this locus in the carcinogenesis introduces two possibilities: (1) 8p22 locus is one of phenotype-determined events tends to develop diffuse gastric cancer; (2) or alternatively, 8p22 locus participate in late stage of diffuse gastric cancer and is more likely to harbor chromosome instabilities. Furthermore, our data allowed us to define a minimal region of allelic loss at 8p22 to a segment around D8S1145 marker with a LOH rate of 53% (Figure 2); make it a good candidate to harbor putative TSG.
Several candidate cancer-susceptibility genes at 8p22, such leucine zipper tumor suppressor 1 (LZTS1) or FEZ1[22-24], deleted in liver cancer DLC1[29] and mitochondrial tumor suppressor gene1 MTUS1[30] are other candidate TSGs in this region. Nevertheless, the minimal region of loss in our tumor samples was telomeric to these genes and the D8S1145 marker with the highest LOH rate is close to the NAT2 locus.
The N-acetyltransferase isoezymes, N-acetyltransferase 1 (NAT1) and N-acetyltransferase 2 (NAT2) catalyze either N-acetylation of aromatic amine and hydrazine drugs or O-acetylation of N-hydroxy-aromatic and heterocyclic amines, and have a primary role in the activation and/or deactivation of a large and diverse number of environmental pollutants[31]. Because NATs activate and/or deactivate environmental pollutants, some of which have been implicated in the etiology of cancers, it has been suggested some polymorphisms that alter the function of NAT genes may be risk factors for the disease[32]. It is often suggested that human NAT2 activity is highest in the liver and gastrointestinal tract[33]. Certain polymorphisms are associated with a decrease in N-acetyltransferase2 activity leading to a possible increased risk factor to develop bladder, gastric, lung and prostate cancers[32-35]. In other hand, occurring of diffuse gastric cancer in proximal gastric tissue is abundance than other section of gastric tissue[36,37] and NAT2 only expressed in this region of stomach[38,39].
Furthermore, accumulating evidences indicate that both genetic and epigenetic changes associate with diffuse gastric cancers[23,40]. Ethnic background suggested being associated with differences in disease aggression and outcome in Asian populations[41,42]. Based on mentioned reports and our LOH rate around NAT2 locus, we hypothesize that the loss of NAT2 gene might influences the progression of this form of cancer or this region harbored another tumor suppressor gene far from of FEZ1 locus.
Further studies will define the key gene targets of alteration on 8p22-23.1 in gastric cancers.
We thank RCGLD personnel for their technical expertise as well as the entire pathology department of Emam Hossoin, Taleghani, Milad, Shohada hospitals that participated in this study.
S- Editor Wang J L- Editor Rampone B E- Editor Chen GJ
| 1. | Chan AO, Luk JM, Hui WM, Lam SK. Molecular biology of gastric carcinoma: from laboratory to bedside. J Gastroenterol Hepatol. 1999;14:1150-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 518] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, Yoonessi A, Tavangar M, Abedi BA, Sotoudehmanesh R. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer. 1997;80:1021-1028. [PubMed] [Cited in This Article: ] |
| 5. | Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1:157-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 647] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 6. | Vogelstein B, Fearon ER, Kern SE, Hamilton SR, Preisinger AC, Nakamura Y, White R. Allelotype of colorectal carcinomas. Science. 1989;244:207-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 898] [Cited by in F6Publishing: 961] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Ribeiro FR, Henrique R, Hektoen M, Berg M, Jerónimo C, Teixeira MR, Lothe RA. Comparison of chromosomal and array-based comparative genomic hybridization for the detection of genomic imbalances in primary prostate carcinomas. Mol Cancer. 2006;5:33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Lu W, Takahashi H, Furusato B, Maekawa S, Ikegami M, Sudo A, Egawa S, Hano H. Allelotyping analysis at chromosome arm 8p of high-grade prostatic intraepithelial neoplasia and incidental, latent, and clinical prostate cancers. Genes Chromosomes Cancer. 2006;45:509-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Bhattacharya N, Chunder N, Basu D, Roy A, Mandal S, Majumder J, Roychowdhury S, Panda CK. Three discrete areas within the chromosomal 8p21.3-23 region are associated with the development of breast carcinoma of Indian patients. Exp Mol Pathol. 2004;76:264-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Venter DJ, Ramus SJ, Hammet FM, de Silva M, Hutchins AM, Petrovic V, Price G, Armes JE. Complex CGH alterations on chromosome arm 8p at candidate tumor suppressor gene loci in breast cancer cell lines. Cancer Genet Cytogenet. 2005;160:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Jin Y, Jin C, Wennerberg J, Höglund M, Mertens F. Cytogenetic and fluorescence in situ hybridization characterization of chromosome 8 rearrangements in head and neck squamous cell carcinomas. Cancer Genet Cytogenet. 2001;130:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Zhou X, Jordan RC, Li Y, Huang BL, Wong DT. Frequent allelic imbalances at 8p and 11q22 in oral and oropharyngeal epithelial dysplastic lesions. Cancer Genet Cytogenet. 2005;161:86-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Ohgaki K, Iida A, Ogawa O, Kubota Y, Akimoto M, Emi M. Localization of tumor suppressor gene associated with distant metastasis of urinary bladder cancer to a 1-Mb interval on 8p22. Genes Chromosomes Cancer. 1999;25:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 14. | Knowles MA, Aveyard JS, Taylor CF, Harnden P, Bass S. Mutation analysis of the 8p candidate tumour suppressor genes DBC2 (RHOBTB2) and LZTS1 in bladder cancer. Cancer Lett. 2005;225:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Liao C, Zhao M, Song H, Uchida K, Yokoyama KK, Li T. Identification of the gene for a novel liver-related putative tumor suppressor at a high-frequency loss of heterozygosity region of chromosome 8p23 in human hepatocellular carcinoma. Hepatology. 2000;32:721-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Tonon G, Wong KK, Maulik G, Brennan C, Feng B, Zhang Y, Khatry DB, Protopopov A, You MJ, Aguirre AJ. High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci USA. 2005;102:9625-9630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Flanagan JM, Healey S, Young J, Whitehall V, Trott DA, Newbold RF, Chenevix-Trench G. Mapping of a candidate colorectal cancer tumor-suppressor gene to a 900-kilobase region on the short arm of chromosome 8. Genes Chromosomes Cancer. 2004;40:247-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Baffa R, Santoro R, Bullrich F, Mandes B, Ishii H, Croce CM. Definition and refinement of chromosome 8p regions of loss of heterozygosity in gastric cancer. Clin Cancer Res. 2000;6:1372-1377. [PubMed] [Cited in This Article: ] |
| 19. | Gustafson CE, Wilson PJ, Lukeis R, Baker E, Woollatt E, Annab L, Hawke L, Barrett JC, Chenevix-Trench G. Functional evidence for a colorectal cancer tumor suppressor gene at chromosome 8p22-23 by monochromosome transfer. Cancer Res. 1996;56:5238-5245. [PubMed] [Cited in This Article: ] |
| 20. | Ramshaw IA, Carlsen S, Wang HC, Badenoch-Jones P. The use of cell fusion to analyse factors involved in tumour cell metastasis. Int J Cancer. 1983;32:471-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Liu H, Ye SL, Yang J, Tang ZY, Liu YK, Qin LX, Qiu SJ, Sun RX. The microcell mediated transfer of human chromosome 8 into highly metastatic rat liver cancer cell line C5F. World J Gastroenterol. 2003;9:449-453. [PubMed] [Cited in This Article: ] |
| 22. | Ishii H, Baffa R, Numata SI, Murakumo Y, Rattan S, Inoue H, Mori M, Fidanza V, Alder H, Croce CM. The FEZ1 gene at chromosome 8p22 encodes a leucine-zipper protein, and its expression is altered in multiple human tumors. Proc Natl Acad Sci USA. 1999;96:3928-3933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Vecchione A, Ishii H, Shiao YH, Trapasso F, Rugge M, Tamburrino JF, Murakumo Y, Alder H, Croce CM, Baffa R. Fez1/lzts1 alterations in gastric carcinoma. Clin Cancer Res. 2001;7:1546-1552. [PubMed] [Cited in This Article: ] |
| 24. | Ono K, Uzawa K, Nakatsuru M, Shiiba M, Mochida Y, Tada A, Bukawa H, Miyakawa A, Yokoe H, Tanzawa H. Down-regulation of FEZ1/LZTS1 gene with frequent loss of heterozygosity in oral squamous cell carcinomas. Int J Oncol. 2003;23:297-302. [PubMed] [Cited in This Article: ] |
| 25. | Bielawski K, Zaczek A, Lisowska U, Dybikowska A, Kowalska A, Falkiewicz B. The suitability of DNA extracted from formalin-fixed, paraffin-embedded tissues for double differential polymerase chain reaction analysis. Int J Mol Med. 2001;8:573-578. [PubMed] [Cited in This Article: ] |
| 26. | Sanguinetti CJ, Dias Neto E, Simpson AJ. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques. 1994;17:914-921. [PubMed] [Cited in This Article: ] |
| 27. | Canzian F, Salovaara R, Hemminki A, Kristo P, Chadwick RB, Aaltonen LA, de la Chapelle A. Semiautomated assessment of loss of heterozygosity and replication error in tumors. Cancer Res. 1996;56:3331-3337. [PubMed] [Cited in This Article: ] |
| 28. | Lu Y, Yu Y, Zhu Z, Xu H, Ji J, Bu L, Liu B, Jiang H, Lin Y, Kong X. Identification of a new target region by loss of heterozygosity at 5p15.33 in sporadic gastric carcinomas: genotype and phenotype related. Cancer Lett. 2005;224:329-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Zhang Q, Ying J, Zhang K, Li H, Ng KM, Zhao Y, He Q, Yang X, Xin D, Liao SK. Aberrant methylation of the 8p22 tumor suppressor gene DLC1 in renal cell carcinoma. Cancer Lett. 2007;249:220-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Di Benedetto M, Pineau P, Nouet S, Berhouet S, Seitz I, Louis S, Dejean A, Couraud PO, Strosberg AD, Stoppa-Lyonnet D. Mutation analysis of the 8p22 candidate tumor suppressor gene ATIP/MTUS1 in hepatocellular carcinoma. Mol Cell Endocrinol. 2006;252:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Deguchi M, Yoshida S, Kennedy S, Ohara N, Motoyama S, Maruo T. Lack of association between endometriosis and N-acetyl transferase 1 (NAT1) and 2 (NAT2) polymorphisms in a Japanese population. J Soc Gynecol Investig. 2005;12:208-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Morton LM, Schenk M, Hein DW, Davis S, Zahm SH, Cozen W, Cerhan JR, Hartge P, Welch R, Chanock SJ. Genetic variation in N-acetyltransferase 1 (NAT1) and 2 (NAT2) and risk of non-Hodgkin lymphoma. Pharmacogenet Genomics. 2006;16:537-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat Res. 2002;506-507:65-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 34. | García-Closas M, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, Tardón A, Serra C, Carrato A, García-Closas R. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 439] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 35. | Tamer L, Calikoğlu M, Aras Ateş N, Yildirim H, Karakaş S, Atik U. Relationship between N-acetyl transferase-2 gene polymorphism and risk of bronchial asthma. Tuberk Toraks. 2006;54:137-143. [PubMed] [Cited in This Article: ] |
| 36. | van Dekken H, Alers JC, Riegman PH, Rosenberg C, Tilanus HW, Vissers K. Molecular cytogenetic evaluation of gastric cardia adenocarcinoma and precursor lesions. Am J Pathol. 2001;158:1961-1967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Eskandar H, Hossein SS, Rahim M, Jalal H, Mehrdad A, Rajabi T. Clinical profile of gastric cancer in Khuzestan, southwest of Iran. World J Gastroenterol. 2006;12:4832-4835. [PubMed] [Cited in This Article: ] |
| 38. | Hickman D, Pope J, Patil SD, Fakis G, Smelt V, Stanley LA, Payton M, Unadkat JD, Sim E. Expression of arylamine N-acetyltransferase in human intestine. Gut. 1998;42:402-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Windmill KF, Gaedigk A, Hall PM, Samaratunga H, Grant DM, McManus ME. Localization of N-acetyltransferases NAT1 and NAT2 in human tissues. Toxicol Sci. 2000;54:19-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Davis PA, Sano T. The difference in gastric cancer between Japan, USA and Europe: what are the facts? what are the suggestions? Crit Rev Oncol Hematol. 2001;40:77-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Theuer CP, Kurosaki T, Ziogas A, Butler J, Anton-Culver H. Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer. 2000;89:1883-1892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 42. | Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a canadian cancer center. J Clin Oncol. 2003;21:2070-2076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |