Published online May 21, 2007. doi: 10.3748/wjg.v13.i19.2761
Revised: December 11, 2006
Accepted: March 19, 2007
Published online: May 21, 2007
Von Recklinghausen’s disease is an autosomal dominant hereditary disease associated with a wide number of neoplasms. We report a case of a 47-year-old Caucasian male affected by Von Recklinghausen’s disease who developed a malignant somatostatinoma of the papilla major and minor associated with jejunal gastrointestinal stromal tumour with uncertain behaviour. At laparotomy, multiple hepatic metastases were evident. Whipple pancreaticoduodenectomy, jejunal resection, extensive lymphadenectomy and multiple hepatic wedge resections were performed. The patient was alive without recurrence after 24 mo. This is the fourth case reported in the world literature of a patient with Von Recklinghausen’s disease associated with periampullary somatostatinomas and jejunal stromal tumor. In patients with Von Recklinghausen’s disease who complain of gastrointestinal symptoms, a high suspicion index for periampullary endocrine tumours and/or gastrointestinal stromal tumour is required. An aggressive surgical approach seems to give long term survival also in metastatic patients.
- Citation: Bettini R, Falconi M, Crippa S, Capelli P, Boninsegna L, Pederzoli P. Ampullary somatostatinomas and jejunal gastrointestinal stromal tumor in a patient with Von Recklinghausen’s disease. World J Gastroenterol 2007; 13(19): 2761-2763
- URL: https://www.wjgnet.com/1007-9327/full/v13/i19/2761.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i19.2761
Von Recklinghausen’s disease (VRD), also known as type 1 neurofibromatosis, is an autosomal dominant disorder with variable penetrance, occurring in about 1 per 3000 births. The gene for VRD has been identified on chromosome 17 and approximately 50% of the cases present as new mutations[1].
The disease is clinically characterized by multiple cutaneous neurofibromas, café-au-lait spots, axillary or inguinal freckling, Lisch nodules (pigmented iris hamartomas). Von Recklinghausen’s disease is also known to be associated with a variety of neoplastic lesions with a neurogenic or neuroendocrine origin and 3%-15% of those patients will develop malignant tumours during their life[2]. In the last 20 years periampullary tumours have been recognized with increased incidence in patients with VRD. Neurofibromas and endocrine tumours are the most common tumours of the papilla of Vater complicating VRD[2,3].
We report a case of a patient with VRD presenting a so called malignant “somatostatinoma” of the papilla major and minor associated with a jejunal gastrointestinal stromal tumour (GIST) with uncertain behaviour.
A 47-year-old Caucasian male affected by VRD was admitted to our Department in April 2004 with a presumptive diagnosis of duodenal carcinoids.
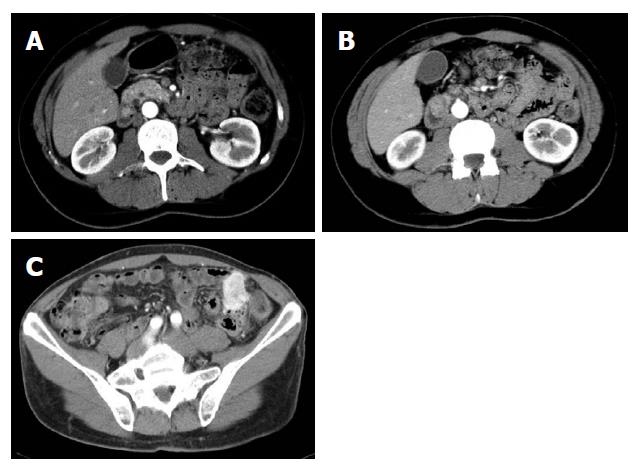
His medical history started in September 2002 when he was admitted to another hospital because of melena. Upper and lower gastrointestinal tract endoscopy showed a Barrett disease and a specific medical treatment was started. The patient was well until December 2003 when melena associated with diarrhoea recurred. Gastroduodenoscopy showed two submucosal ulcerated lesions on papilla major and minor which were diagnosed as carcinoids based on histological examination. Common and endocrine tumour markers (NSE, CgA, CEA, CA 19.9) as well as laboratory routine tests were within normal values. Gastrin levels were high (267 pg/mL) but secretin test was negative. A small bowel barium follow through and an abdominal computed tomography (CT) scan raised the suspicion for another hypervascularized lesion in the distal jejunum (Figure 1A-C) while an octreoscan was negative for all aforementioned lesions.
In April 2004 the patient was sent to our Department for a surgical approach. Physical examination revealed multiple generalized cutaneous neurofibromas of varying sizes and café-au-lait skin spots. Skin was not jaundiced, no lymphadenopathy was palpable, abdominal examination was unremarkable. An ultrasound sonography (US) with contrast medium was negative for liver metastases.
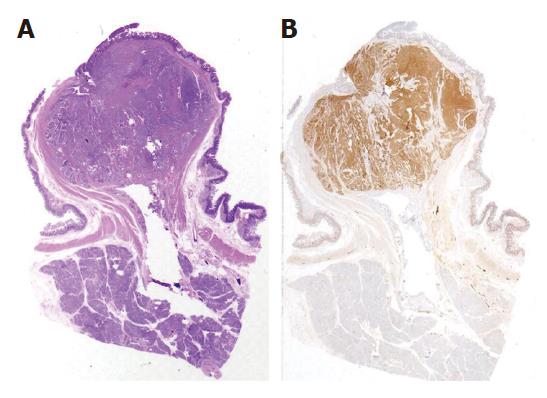
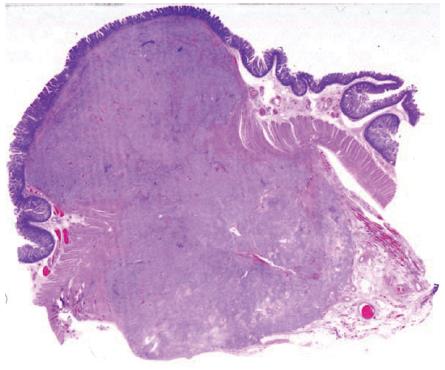
At laparotomy three hepatic metastases were seen on liver surface. An intraoperative histological examination of the hepatic lesions was positive for metastases from endocrine carcinoma. After an intraoperative ultrasound (IOUS) assessment, another three lesions were seen and judged as respectable. Small bowel examination confirmed the jejunal lesion. Whipple pancreaticoduodenectomy with jejunal resection, extensive lymphadenectomy and multiple hepatic wedge resections were performed with radical intent. Moreover, one cutaneous lesion was excised. On opening the resected specimen two whitish, nodular lesions measuring 1.7 cm and 1.2 cm were seen in papilla major and minor associated with a jejunal brownish lesion, 7 cm in size, with exophytic growth. Final pathological examination established a papilla major and minor well-differentiated endocrine carcinoma with strong immunohistochemical expression of somatostatin (“somatostatinoma”) and ki67 < 1 % (Figure 2A and B) associated with nodal metastases in 6 out of 73 resected nodes (2 out of 10 mesenteric nodes). Moreover, 6 liver metastases measuring from 0.5 to 2 cm in size from an endocrine carcinoma were diagnosed. Considering the jejunal neoplasm, a diagnosis of GIST with uncertain behaviour (major diameter 6 cm, CD117++, mitoses: 2/10 high power fields) was made (Figure 3). The cutaneous lesion was a neurofibroma with a proliferation of all the elements in the peripheral nerve including neuritis, Schwann cells and fibroblasts.
The postoperative course was uneventful. The patient was discharged 9 days after operation. He was alive without recurrent disease 16 mo after the initial surgery.
VRD is an autosomal dominant hereditary disease due to a mutation of the NF1 gene on chromosome 17. This gene encodes a GTPase activating protein that can regulate the activity of the p21 product of the ras oncogene and seems to play an important role in controlling cell proliferation and differentiation in a wide range of tissues. Different mutations in the gene are involved in the development of various clinical manifestations of VRD, including malignant tumours[5].
The patient developed at the same time three tumours: one jejunal GIST and two endocrine neoplasms (one papilla major neoplasm and one papilla minor neoplasm), with strong immunohystochemical expression of somatostatin (“somatostatinoma”). This is the forth case described in the world literature of a VRD patient associated with this rare somatostatinoma[6-8].
Gastrointestinal (GI) lesions reported in about 25% of patients affected by VRD, can be divided into three groups: (1) hyperplasia of the gut neural tissue and its supporting structures leading to abnormalities of GI motility, (2) endocrine tumours of the duodenum and the periampullary region, (3) GIST with different degrees of neural and smooth muscle differentiation and biological behaviour[2-4,9].
In VRD, endocrine tumours of the periampullary region show a glandular architecture, contain calcified psammoma bodies, sometimes produce somatostatin and are positive for somatostatin at immunohystochemical analysis. Usually they are not functioning and symptoms are related to local mass effects such as obstructive jaundice, haemorrhage, intestinal obstruction, and cholangitis[2]. It has been supposed that in such cases tumor-like symptoms appear before enough somatostatin is produced to cause the related syndrome[10,11]. To the best of our knowledge, from 1981, only 34 cases of “so called” periampullary somatostatinoma associated with VRD have been reported. The patients’ age ranged from 21 to 70 years, with an equal distribution between sexes. Jaundice and abdominal pain are the most common symptoms, while manifestations of the somatostatinoma syndrome (diabetes mellitus, steatorrhea and cholelithiasis) can be found in only few patients. In 56% of patients the greatest diameter was 2 cm or less. The resection of choice was a pancreaticoduodenectomy (60%) while in the remaining cases a local resection was performed[6-10]. This latter procedure is considered as an appropriate treatment for small duodenal tumours (< 2 cm) far from the ampulla[6,7]. Nevertheless regional node involvement has been reported in 53% of patients even when the tumour was less than 2 cm in size[12]. These data and the possible invasion of the deepest layers make, in our opinion, mandatory pancreaticoduodenectomy as a procedure of choice.
The role of surgery in patients with distant metastases is still under debate[11]. Patel et al[10] have reported long term disease free survival with good quality of life in patients who underwent radical resection both of the primary tumour and liver metastases. However, the optimal surgical choice is unknown because of the small number of cases collected and the lack of randomized controlled trials. In regard to prognosis, although long term follow-up is available in only few cases, the postoperative survival ranged from 4 to 60 mo (mean 18.4, median 14.5) and only two patients died of the disease[6-10].
In regard to GIST, its incidence in VRD ranges from 4% to 25%. GISTs are neoplasms characterized by CD117 positive mesenchymal spindle, epithelioid or pleomorphic cells[3,13-15], often found incidentally during unrelated surgery or autoptic studies. On the other hand, they may be symptomatic, causing obstruction, volvulus, intussusception, ulceration, bleeding and perforation[2,13,15]. Most of the GISTs associated with VRD are benign or with uncertain biological behaviour while malignant tumours are uncommon. Histologically there are no specific differences between GISTs associated with VRD and those occurring in non-VRD patients. However, tumours associated with VRD frequently show jejunal location, multiplicity, abundant skeinoid fibres and sometimes a partial ultrastructural neural differentiation.
Moreover, Griffiths et al[4] demonstrated that patients with VRD and duodenal endocrine tumours have a high risk of developing phaeochromocytoma and the frequency of phaeochromocytoma is about 1% in VRD patients but increases up to 22% in those with associated endocrine duodenal tumours. However, no evidence of phaeochromocytoma was found in this case.
In conclusion, in patients with VRD who complain of gastrointestinal symptoms, a high suspicion index of periampullary endocrine tumours and/or GISTs is required. An aggressive surgical approach seems to give long-term survival and good palliation also in metastatic patients.
S- Editor Liu Y L- Editor Wang XL E- Editor Chen GJ
| 1. | Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med. 1981;305:1617-1627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 836] [Cited by in F6Publishing: 861] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 2. | Behranwala KA, Spalding D, Wotherspoon A, Fisher C, Thompson JN. Small bowel gastrointestinal stromal tumours and ampullary cancer in Type 1 neurofibromatosis. World J Surg Oncol. 2004;2:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease). Histopathology. 1991;19:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 197] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Griffiths DF, Williams GT, Williams ED. Duodenal carcinoid tumours, phaeochromocytoma and neurofibromatosis: islet cell tumour, phaeochromocytoma and the von Hippel-Lindau complex: two distinctive neuroendocrine syndromes. Q J Med. 1987;64:769-782. [PubMed] [Cited in This Article: ] |
| 5. | Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O'Connell P, Cawthon RM. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 700] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 6. | Suzuki S, Sato K, Katada E, Kuno Y, Mizoguchi N, Tokuda H, Inaguma S, Asamoto M, Dohi Y. Periampullary somatostatinoma and multiple gastrointestinal stromal tumors associated with von Recklinghausen's disease. J Gastroenterol. 2004;39:1011-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Usui M, Matsuda S, Suzuki H, Hirata K, Ogura Y, Shiraishi T. Somatostatinoma of the papilla of Vater with multiple gastrointestinal stromal tumors in a patient with von Recklinghausen's disease. J Gastroenterol. 2002;37:947-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Karatzas G, Kouraklis G, Karayiannakis A, Patapis P, Givalos N, Kaperonis E. Ampullary carcinoid and jejunal stromal tumour associated with von Recklinghausen's disease presenting as gastrointestinal bleeding and jaundice. Eur J Surg Oncol. 2000;26:428-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Pinsk I, Dukhno O, Ovnat A, Levy I. Gastrointestinal complications of von Recklinghausen's disease: two case reports and a review of the literature. Scand J Gastroenterol. 2003;38:1275-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Patel VG, Henderson VJ, Fairweather DA, Fortson JK, Weaver WL, Martin DM, Lyons R, Hamami A. Malignant duodenal somatostatinoma presenting in association with von Recklinghausen disease. Am Surg. 2003;69:1077-1082. [PubMed] [Cited in This Article: ] |
| 11. | Delcore R, Friesen SR. Gastrointestinal neuroendocrine tumors. J Am Coll Surg. 1994;178:187-211. [PubMed] [Cited in This Article: ] |
| 12. | Ricci JL. Carcinoid of the ampulla of Vater. Local resection or pancreaticoduodenectomy. Cancer. 1993;71:686-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 13. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1185] [Cited by in F6Publishing: 1127] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 14. | Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 809] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 15. | Takazawa Y, Sakurai S, Sakuma Y, Ikeda T, Yamaguchi J, Hashizume Y, Yokoyama S, Motegi A, Fukayama M. Gastrointestinal stromal tumors of neurofibromatosis type I (von Recklinghausen's disease). Am J Surg Pathol. 2005;29:755-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |