Published online Mar 21, 2007. doi: 10.3748/wjg.v13.i11.1728
Revised: December 28, 2006
Accepted: February 25, 2007
Published online: March 21, 2007
AIM: To investigate the pRb expression in a large group of patients with history of chronic exposure to the main risk factors for development of squamous cell carcinoma of the esophagus.
METHODS: One hundred and seventy asympto-matic individuals at high risk for esophageal squamous cell carcinoma (consumption of more than 80 g of ethanol and 10 cigarettes/d for at least 10 years) underwent upper gastrointestinal endoscopy with biopsies of the esophageal mucosa. As a control group, specimens of esophageal mucosa obtained from 20 healthy subjects were also studied. Immunohistochemical assessment of the tissues was performed using a monoclonal antibody anti-pRB protein.
RESULTS: Absence of the pRB staining, indicating loss of RB function, was observed in 33 (19.4%) of the individuals at risk for esophageal cancer, but in none of the healthy controls (P < 0.02). Loss of pRb expression increased in a stepwise fashion according to the severity of the histological findings (P < 0.005): normal mucosa (11/97 or 11.3%), chronic esophagitis (17/60 or 28.3%), low-grade dysplasia (3/10 or 30%), high-grade dysplasia 1/2 or 50%) and squamous cell carcinoma (1/1 or 100%).
CONCLUSION: Our findings suggest that abnormal expression of the pRB protein may be implicated in the process of esophageal carcinogenesis. Additional studies are warranted to define the role of the pRB protein as a biomarker for development of esophageal squamous cell carcinoma in individuals at high risk for this malignancy.
- Citation: Contu SS, Contu PC, Damin DC, Fagundes RB, Bevilacqua F, Rosa AS, Prolla JC, Moreira LF. pRB expression in esophageal mucosa of individuals at high risk for squamous cell carcinoma of the esophagus. World J Gastroenterol 2007; 13(11): 1728-1731
- URL: https://www.wjgnet.com/1007-9327/full/v13/i11/1728.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i11.1728
In most countries, the incidence of esophageal squamous cell carcinoma (ESCC) varies between 2.5 and 5.0/100 000 for men and between 1.5 and 2.5 for women[1]. Brazil is one of the high-risk countries for esophageal cancer. Particularly, Rio Grande do Sul (Southern Brazil) is the state with the highest annual incidence in the country, approximately 22.5 cases/100 000 for men and 7.5/100 000 for women.
Although several risk factors for development of oesophageal carcinoma have been identified, such as smoking and alcohol consumption, the molecular mechanisms related to the oesophageal carcinogenesis remain under investigation[2]. In this context, deregulations of the cell-cycle-controlling mechanisms, RB-pathway in particular, have been suggested to be involved in the pathogenesis of oesophageal cancer[3].
The RB gene, located on chromosome 13q14, encodes a 110-kd key nuclear phosphoprotein implicated in the regulation of the transcription control mechanisms mediating progression throughout G1 phase of the cell-cycle[4]. Inactivation of pRB by different mechanisms (homozygous deletion, promoter hypermethylation or point mutations within the coding sequence) leads to a release of the pRB-bound E2F-members and transcription of the S-phase genes, promoting malignant transformation[5,6].
Abnormal pRB expression has been reported in different malignant tumours, such as lung, bladder and prostate carcinomas[7-10]. In oesophageal cancer, decreased pRB immunohistochemical nuclear staining indicating loss of RB gene function has been reported to occur in a high proportion of cases[11-14]. To date, however, there is no published study investigating the pRb expression in oesophageal mucosa of asymptomatic individuals from a population at high risk for cancer of the esophagus. The aim of present study was, therefore, to evaluate the pRb expression in a large group of patients with history of chronic exposure to the main risk factors for development of ESCC.
This study consisted of 170 male patients (mean age 45 years, range 35 to 69 years) treated at the Drug Abuse Unit of the Psychiatric University Hospital (Santa Maria Federal University) and the Drug Abuse Support Group of the city of Santa Maria. Ninety percent of the patients were white. All patients presented a past history of alcohol abuse (80 g of ethanol per day for more than 10 years), being in abstinence for a period of less than twelve months. They also reported regular consumption of tobacco (more than 10 cigarettes per day for more than 10 years) and daily drinking of hot tea (Paraguayan-mate tea for more than 10 years). None of them presented digestive symptoms.
As a control group, 20 healthy volunteers were studied. There were six males and 14 females with mean age of 45 years (range 29 to 61 years). All controls were white. None of them was an alcohol, tobacco or mate tea consumer or had family history of cancer.
The study was performed after approval by the Ethics and Scientific Committees of the Santa Maria Federal University. Informed consent was obtained from all patients and controls before being enrolled in the study.
Patients were investigated by a conventional upper gastrointestinal endoscopy followed by chromoendoscopic examination with 3% lugol. Biopsies were obtained from any visible lesion and lugol unstained areas greater than 5 mm in diameter. The collected tissue samples were stained with haematoxylin-eosin (HE) and submitted to histological examination. Histolopathologic diagnoses of the tissues obtained from the 170 patients at risk for esophageal cancer were: 97 normal mucosas (57%), 57 mild or moderate chronic esophagitis (33.5%), three severe chronic esophagitis (1.8%), 10 low-grade dysplasias (5.9%), two high-grade dysplasias (1.2%) and one squamous cell carcinoma (0.6%)[15].
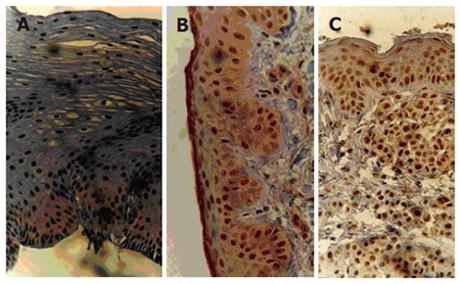
Immunohistochemical assessment of the 190 paraffin-embedded tissues was performed using a monoclonal antibody anti-pRB protein (DAKO Lab., New York, NY, USA) diluted at 1:25. To determine the antibody reactivity, the avidin-biotin peroxidase complex (ABC method; kit LSAB DAKO) was used. The procedure was carried out at the Pathology Unit of the Hospital de Clínicas de Porto Alegre, Federal University of Rio Grande do Sul. Positive cases were identified through visualization of a strong brownish nuclear granulation stained by the diaminobenzidine (DAB) chromogen. As positive controls for the immunohistochemical reaction, breast carcinomas known to express pRb were used. Reactions conducted without the primary antibody were used as negative controls. Cases were identified as positive if more than 5% of the cells in a 400 magnification microscopic field showed nuclear accumulation of pRB protein (Figure 1)[11,15]. Each slide was analyzed by two independent pathologists.
Variables were described by mean and standard error. Correlation between clinicopathological variables and loss of pRB immunohistochemical expression was evaluated by univariate analysis using chi-square or Fisher exact test. Logistic regression was used to calculate odds ratio and confidence intervals, with multivariate adjustment for potential confounding variables. Statistical significance was accepted at the 5% level.
Nuclear accumulation of pRB protein was observed in 137 (80.6%) of the 170 patients at risk for ESCC. Loss of the pRB staining was observed in 33 (19.4%) of those patients, but in none of the 20 healthy controls (P < 0.02).
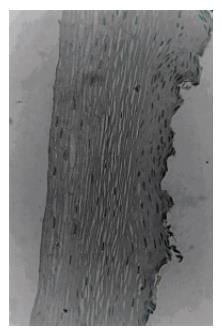
The pRB immunoreaction was negative in 30% (21/69) of the patients with a family history of cancer as compared with only 12% (12/101) of those without family history (P < 0.005). There was no significant correlation between the pRB status and age or race of the subjects (Table 1). No significant correlation between pRB expression and period or volume of alcohol, tobacco or mate tea consumption was detected. In contrast, it was observed a linear correlation between the histological classification of the tissues and the absence of nuclear pRB staining (P < 0.001). Loss of nuclear pRB expression significantly increased with the progression of the histopathologic alterations as follows: normal mucosa (11/97 or 11.3%), chronic esophagitis (17/60 or 28.3%), low-grade dysplasia (3/10 or 30%), high-grade dysplasia (1/2 or 50%) and squamous cell carcinoma (1/1 or 100%). Figure 1 presents the histological aspect of the positive cases after immunohistochemical staining, including examples of chronic esophagitis, low-grade dysplasia and squamous cell carcinoma. Figure 2 shows an example of normal esophageal tissue sample without nuclear accumulation of pRB protein.
| Characteristics | pRB – (%) | pRB + (%) | Total | P |
| Age (yr) | ||||
| Under 50 | 10 (22.7) | 34 (77.3) | 44 | |
| Over 50 | 23 (18.3) | 103 (81.7) | 126 | NS |
| Race | ||||
| Caucasian | 31 (20.0) | 124 (80.0) | 155 | |
| Others | 2 (13.3) | 13 (86.7) | 15 | NS |
| Family history of cancer | ||||
| negative | 12 (11.9) | 89 (88.1) | 101 (59.4) | |
| positive | 21 (30.4) | 48 (69.6) | 69 (41.6) | 0.005 |
In Table 2, we present the odds ratio for loss of pRB immunohistochemical expression calculated by the logistic regression model. Family history of cancer and histopathologic classification were significantly associated with an increased risk for loss of pRB expression.
| Feature | OR | 95% CI | P |
| Familial history of cancer | 3.83 | 1.62-9.04 | 0.0021 |
| Age | 1.28 | 0.52-3.15 | NS |
| Race | 0.72 | 0.15-3.52 | NS |
| Histology | 3.98 | 0.90-17.4 | NS |
| Lugol staining | NS | NS | NS |
Endoscopic examination was apparently normal in all healthy controls. However, histological examination of their tissue samples revealed normal mucosa in 75% of cases and mild chronic esophagitis in 25%. All controls were positive for pRb staining.
Despite recent improvement on surgical techniques and adjuvant therapies, the prognosis of ESCC remains grim. The outcome of patients with advanced disease, the most common clinical presentation, is still very poor, and survival rates in five years rarely exceed 5%-10%[16]. ESCC is high prevalent in Southern Brazil, where alcohol consumption, tobacco use and hot “mate” drinking have been implicated as risk factors. The mortality rate for ESCC in this region is 14.3 and 4.2 for 100.000 inhabitants for men and women respectively[17].
Multiple stages of esophageal carcinogenesis have been defined histologically. It has been established that esophageal carcinoma usually evolves through a series of progressively severe histopathologic changes which involves dysplasia of the epithelium, carcinoma in situ and, finally, invasive carcinoma[15,18]. Based on the concept that precancerous cells may have early alterations at the molecular level, identification of new potential biomarkers might play an important role in detection of high-risk lesions for malignant transformation and early diagnosis of ESCC.
Several genetic alterations have been reported in ESCC, such as expression of proto-oncogenes, cyclin D1 amplification, allelic losses and gene mutations[3]. Inactivation of the RB gene has been shown in a variety of human cancers, including ESCC[5-9]. On the protein level, Jiang[11] demonstrated loss of pRB expression in 17% of ESCC, while other researchers reported a loss of pRB expression in up to 31% of cases[8,14].
Zur Hausen and colleagues analyzed the pRB expression in samples of normal esophageal epithelium (n = 10), severe dysplasia (n = 19), carcinoma in situ (n = 14), invasive ESCC (n = 172), and two continuous esophageal-carcinoma cell lines by immunohistochemistry. In normal esophageal epithelium, nuclear pRB expression was restricted to the parabasal cell layer, whereas, in a considerable portion of severe dysplasias and carcinomas in situ, pRb over-expression was found. Among carcinomas, 161 of 172 cases showed pRb expression, as did the 2 esophageal-carcinoma cell lines, whereas 11 carcinomas were negative. Expression of pRB among carcinomas was not correlated with pT category, pN category or tumor grade. In the univariate survival analysis, patients with pRB-negative tumors showed lower 2-year and 5-year survival rates (27.3%/9.1%) than patients with pRB-positive tumors (42.8%/25.8%; not significant)[12].
Ikeguchi and colleagues[19] studied surgically resected ESCC through immunohistochemical analysis for altered pRB expression. Decreased pRB nuclear staining indicating loss of RB function occurred in 43% of the cases studied. The incidence of decreased pRB expression was higher in tumors with invasion to the adventitia (50%) than in tumors without invasion to the adventitia (33%, P = 0.0188). In addition, the incidence of decreased pRB expression was higher in tumors with lymph node metastasis (50%) than in those without (34%, P = 0.0346). The 3-year survival rates of 82 patients who had tumors with decreased pRB expression (30%) was significantly lower than that of 109 patients who had tumors with normal pRB expression (52%, P = 0.0032). However, in the multivariate survival analysis, pRB expression was not an independent prognostic factor for patients with esophageal squamous cell carcinoma.
To the best of our knowledge, this is the first study investigating the pRB immunohistochemical expression in esophageal mucosa of asymptomatic individuals who have been chronically exposed to the main risk factors for ESCC. We demonstrated the loss of the pRb expression in 19.4% of the individuals at risk for ESCC, but in none of the healthy controls. These results suggest that abnormal expression of the pRB protein may be implicated in the process of esophageal carcinogenesis in individuals at high risk for development of ESCC.
We were able to demonstrate a linear correlation between the histological classification of the tissues and the absence of nuclear pRB staining (P < 0.001), from normal mucosa (11.3%) to squamous cell carcinoma (100%), indicating that inactivation of pRB might be involved not only in the initiation of ESCC but also in its progression. Similar results were reported by Xu et al[9] in study of lung carcinomas. RB protein expression was examined in paraffin and frozen tissue sections of 36 primary non-small cell lung carcinomas using immunohistochemistry. A normal RB protein staining pattern was present in 24 and absent in 10 carcinomas. Two additional RB positive primary tumors have major foci in which all tumor cells showed no pRB staining. Significantly more high-stage (stages III and IV) carcinomas had altered pRB expression than those with low-stage (stagesIand II) tumors (P < 0.05). The authors concluded that absence of the pRB expression might be associated with the initiation and/or progression of non-small cell lung carcinomas. Ishikawa and colleagues conducted a similar study in specimens of bladder carcinoma[7]. None of their 16 low-grade noninvasive bladder carcinomas showed an alteration in RB protein by direct Western blot analysis, whereas 2 of 14 high-grade invasive tumors had no pRB as measured by both Western blotting and immunohistochemical staining.
An original finding of the present study was the detection of an association between loss of pRB staining and positive family history of cancer. This point to a potential hereditary predisposition to abnormal pRB expression, which can have influence on the individual susceptibility to developing ESCC in association with chronic exposure to major risk factors for the disease.
We considered as positive those samples in which more than 5% of epithelial basal cells were immunoreactive, since in normal esophageal epithelium pRB protein is restricted to basal and parabasal layers[14]. It must be pointed out, however, that the anti-pRB monoclonal antibody does not differentiate the hyperphosphorilated protein from the hypophosphorilated form. Because the former corresponds to the biologically inactive form, its detection may potentially overestimate the protein expression and function in the pRB-positive group[14,19].
In conclusion, we were able to present evidence for an association between loss of pRB expression and esophageal carcinogenesis. Our data suggest that the RB protein might be useful as biomarker of ESCC in individuals at high risk for this malignancy. It must pointed out, however, that these observations need to be substantiated with additional follow-up studies analyzing a larger number of individuals with high-grade dysplasia and squamous cell carcinoma of the esophagus.
S- Editor Liu Y L- Editor Glaser SS E- Editor Chin GJ
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2651] [Cited by in F6Publishing: 2570] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 2. | Ribeiro U, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1174-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Kawakubo H, Ozawa S, Ando N, Kitagawa Y, Mukai M, Ueda M, Kitajima M. Alterations of p53, cyclin D1 and pRB expression in the carcinogenesis of esophageal squamous cell carcinoma. Oncol Rep. 2005;14:1453-1459. [PubMed] [Cited in This Article: ] |
| 4. | Wiman KG. The retinoblastoma gene: role in cell cycle control and cell differentiation. FASEB J. 1993;7:841-845. [PubMed] [Cited in This Article: ] |
| 5. | Hamel PA, Phillips RA, Muncaster M, Gallie BL. Speculations on the roles of RB1 in tissue-specific differentiation, tumor initiation, and tumor progression. FASEB J. 1993;7:846-854. [PubMed] [Cited in This Article: ] |
| 6. | Weinberg RA. Tumor suppressor genes. Science. 1991;254:1138-1146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1050] [Cited by in F6Publishing: 1143] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 7. | Ishikawa J, Xu HJ, Hu SX, Yandell DW, Maeda S, Kamidono S, Benedict WF, Takahashi R. Inactivation of the retinoblastoma gene in human bladder and renal cell carcinomas. Cancer Res. 1991;51:5736-5743. [PubMed] [Cited in This Article: ] |
| 8. | Cordon-Cardo C, Wartinger D, Petrylak D, Dalbagni G, Fair WR, Fuks Z, Reuter VE. Altered expression of the retinoblastoma gene product: prognostic indicator in bladder cancer. J Natl Cancer Inst. 1992;84:1251-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 264] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Xu HJ, Hu SX, Cagle PT, Moore GE, Benedict WF. Absence of retinoblastoma protein expression in primary non-small cell lung carcinomas. Cancer Res. 1991;51:2735-2739. [PubMed] [Cited in This Article: ] |
| 10. | Bookstein R, Rio P, Madreperla SA, Hong F, Allred C, Grizzle WE, Lee WH. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad SciUSA. 1990;87:7762-7766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 231] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Jiang W, Zhang YJ, Kahn SM, Hollstein MC, Santella RM, Lu SH, Harris CC, Montesano R, Weinstein IB. Altered expression of the cyclin D1 and retinoblastoma genes in human esophageal cancer. Proc Natl AcadSciUSA. 1993;90:9026-9030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 234] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | zur Hausen A, Sarbia M, Heep H, Willers R, Gabbert HE. Retinoblastoma-protein (prb) expression and prognosis in squamous-cell carcinomas of the esophagus. Int J Cancer. 1999;84:618-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 13. | Maesawa C, Tamura G, Suzuki Y, Ogasawara S, Ishida K, Saito K, Satodate R. Aberrations of tumor-suppressor genes (p53, apc, mcc and Rb) in esophageal squamous-cell carcinoma. Int J Cancer. 1994;57:21-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Busatto G, Shiao YH, Parenti AR, Baffa R, Ruol A, Plebani M, Rugge M. p16/CDKN2 alterations and pRb expression in oesophageal squamous carcinoma. Mol Pathol. 1998;51:80-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Dawsey SM, Lewin KJ, Liu FS, Wang GQ, Shen Q. Esophageal morphology from Linxian, China. Squamous histologic findings in 754 patients. Cancer. 1994;73:2027-2037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Koshy M, Esiashvilli N, Landry JC, Thomas CR, Matthews RH. Multiple management modalities in esophageal cancer: epidemiology, presentation and progression, work-up, and surgical approaches. Oncologist. 2004;9:137-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Muñoz N, Victora CG, Crespi M, Saul C, Braga NM, Correa P. Hot maté drinking and precancerous lesions of the oesophagus: an endoscopic survey in southern Brazil. Int J Cancer. 1987;39:708-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Chang-Claude JC, Wahrendorf J, Liang QS, Rei YG, Muñoz N, Crespi M, Raedsch R, Thurnham DI, Correa P. An epidemiological study of precursor lesions of esophageal cancer among young persons in a high-risk population in Huixian, China. Cancer Res. 1990;50:2268-2274. [PubMed] [Cited in This Article: ] |
| 19. | Ikeguchi M, Oka S, Gomyo Y, Tsujitani S, Maeta M, Kaibara N. Clinical significance of retinoblastoma protein (pRB) expression in esophageal squamous cell carcinoma. J Surg Oncol. 2000;73:104-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |