Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.703
Revised: August 9, 2005
Accepted: August 26, 2005
Published online: February 7, 2006
AIM: To estimate whether STI571 inhibits the expression of vascular endothelial growth factor (VEGF) in the gastrointestinal stromal tumor (GIST) cells.
METHODS: We used GIST cell line, GIST-T1. It has a heterogenic 57-bp deletion in exon 11 to produce a mutated c-KIT, which results in constitutive activation of c-KIT. Cells were treated with/without STI571 or stem cell factor (SCF). Transcription and expression of VEGF were determined by RT-PCR and flow cytometry or Western blotting, respectively. Activated c-KIT was estimated by immunoprecipitation analysis. Cell viability was determined by MTT assay.
RESULTS: Activation of c-KIT was inhibited by STI571 treatment. VEGF was suppressed at both the transcriptional and translational levels in a temporal and dose-dependent manner by STI571. SCF upregulated the expression of VEGF and it was inhibited by STI571. STI571 also reduced the cell viability of the GIST-T1 cells, as determined by MTT assay.
CONCLUSION: Activation of c-KIT in the GIST-T1 regulated the expression of VEGF and it was inhibited by STI571. STI571 has antitumor effects on the GIST cells with respect to not only the inhibition of cell growth. but also the suppression of VEGF expression.
- Citation: Jin T, Nakatani H, Taguchi T, Nakano T, Okabayashi T, Sugimoto T, Kobayashi M, Araki K. STI571 (Glivec) suppresses the expression of vascular endothelial growth factor in the gastrointestinal stromal tumor cell line, GIST-T1. World J Gastroenterol 2006; 12(5): 703-708
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/703.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.703
Gastrointestinal stromal tumors (GIST) are characterized by the expression of both c-KIT and CD34 on the cell surface. Mutations in c-kit have recently been implicated with oncogenic activation associated with GISTs[1,2]. c-KIT, a type III receptor tyrosine kinase, is activated upon binding of the ligand, stem cell factor (SCF) to initiate a signaling pathway that is critical for the growth and development of mast cells, melanocytes, hematopoietic stem cells, and the interstitial cells of Cajal[3,4]. Gain-of-function mutations in c-kit are associated with a number of cancers in human beings[1,5-9]. The majority of GISTs show constitutive c-KIT phosphorylation due to a gain-of-function mutation in exon 11 (cytoplasmic juxtamembrane domain), with other mutations known to occur in exon 9 (extracellular membrane domain), exon 13 (first part of the split tyrosine kinase domain), and exon 17 (phosphotransferase domain)[8].
STI571 is a specific tyrosine kinase inhibitor that acts on Bcr-Abl, platelet-derived growth factor receptor (PDGFR), and c-KIT. STI571 has been used successfully in patients with unresectable or metastatic GISTs that show constitutive activation of c-KIT[9].
Vascular endothelial growth factor (VEGF) is a highly specific mitogen for vascular endothelial cells that is induced by hypoxia, oncogene activation, and a variety of cytokines. VEGF is important in angiogenesis and neovascularization of solid tumor growth[10]. Expression of VEGF in GISTs was reported by Takahashi et al [11], who suggested a correlation between this expression and poor prognosis. It is thought that the assessment of VEGF expression in GISTs is important clinically.
In the present study, we have analyzed the expression of VEGF in the GIST cell line, GIST-T1, with or without STI571 treatment. To our knowledge, there are only two GIST cell lines, including GIST-T1. The GIST-T1 line was established from a patient with metastatic GIST[12], which showed the c-kit mutation in exon 11. In addition, we have analyzed the effect of STI571 on cell growth of the GIST-T1 cells.
STI571, also known as Glivec capsule (Novartis, Basel, Switzerland), was dissolved in water (5 μg/μL) and stored at –20 ˚C. Antibodies were used to detect the c-KIT (K963, rabbit polyclonal IgG, Immuno-Biological Laboratories, Japan), phosphotyrosine (PY20, mouse monoclonal IgG, Zymed, USA), HIF-1 alpha (H-206, rabbit polyclonal IgG, Santa Cruz Biotechnology, USA), and VEGF (A-20, rabbit polyclonal IgG, Santa Cruz Biotechnology, USA).
The human GIST cell line, GIST-T1 has been characterized in detail by Taguchi et al [12]. GIST-T1 cells and DLD-1 cells (colon adenocarcinoma cell line) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin, streptomycin, and 80 mL/L fetal bovine serum maintained in a 50 mL/L CO2 atmosphere at 37 ˚C in a humidified incubator.
Cells were washed thrice with ice-cold phosphate buffered saline (PBS) and then lysed in RIPA buffer containing 20 mmol/L sodium pyrophosphate, 20 mmol/L NaF, 1 mmol/L orthovanadate, 2 mmol/L pyrophosphate, 1 mmol/L PMSF, 10 μg/mL aprotinin, and 10 μg/mL leupeptin. Cell lysates containing comparable amounts of proteins, estimated by a Bradford assay (BioRad, Munchen, Germany), were analyzed by Western blotting using antibodies as listed above.
A measure of 4×106 cells was seeded on 10-cm dishes and incubated for 24 h, prior to the treatment with STI571. Cells were treated with or without STI571 (1 μg/mL) for 1 h. Treated cells were collected and washed with ice-cold PBS thrice and lysed in RIPA buffer as described above. Cells lysates in RIPA buffer were subjected to immunoprecipitation with c-KIT antibody. The immunoprecipitates were reacted with protein A-agarose and washed with Tris buffered saline. They were then finally resuspended in 3×SDS sample buffer containing 30 × DTT and boiled at 95 ˚C for 5 min. Samples were separated by 75 g/L SDS-PAGE and transferred to a membrane for immunoblot analysis.
A measure of 1×106 cells was seeded on 6-cm dishes and incubated for 24 h prior to the treatment with or without the reagents. Prior to the treatment with SCF, medium was changed to the serum-free medium. Treated cells were washed twice in ice-cold PBS and the total cellular RNA was isolated by using TRIzol reagent (Gibco-BRL) according to the manufacturer’s protocol. Specific mRNA was assayed using the reverse transcription polymerase chain reaction (RT-PCR). VEGF PCR condition[13]: denaturation at 94 oC for 1 min, annealing at 60 oC for 1 min and elongation at 72 oC for 1 min, for 35 cycles; HIF-1 alpha[14]: denaturation at 94 oC for 10 min-1 cycle, followed by 35 cycles of 30 s at 94 oC, 30 s at 55 oC and 1 min at 72 oC; beta-actin: denaturing, 94 oC for 30 s, annealing at 50 oC for 40 s and elongation, 72 oC for 1 min, for 30 cycles; and final elongation at 72 oC for 10 min-1 cycle. PCR products were subjected to electrophoresis on 20 g/L agarose gel. The primer sequences were VEGF sense: 5’ -CGAAGTGGTGAAGTTCATGGATG-3’; VEGF antisense: 5’ -TTCTGTATCAGTCTTTCCTGGTGA-3’; HIF-1 alpha sense: 5’ -CTCAAAGTCGGACAGCCTCA-3’; HIF-1 alpha antisense: 5’ - CCCTGCAGTAGGTTTCTGCT-3’; beta-actin sense: 5’-ATTGGCAATGAGCGGTTCCGC-3’; beta-actin antisense: 5’-CTCCTGCTTGCTGATCCACATC-3’.
A measure of 2 × 106 cells was seeded on 6-cm dishes and incubated for 24 h, prior to the treatment with STI571 for 4 h. Following this, a Goldi plug (BD Bioscience, San Diego, CA, USA) was added to each dish for 2-h incubation. Cells were then washed with ice-cold PBS twice before the treatment with cytoplex (BD Bioscience) and cytoperm (BD Bioscience) according to the manufacturer’s protocols. Anti-VEGF, anti-HIF-1 alpha, and FITC-labeled anti-rabbit antibodies were used. Normal rabbit IgG antibody and FITC-labeled anti-rabbit antibody were used for control staining.
A measure of 1 × 104 cells/100 μL was seeded in each well of a 96-well plate. After 24 h with or without STI571 treatment, 100 μL of a 2.5 mg/mL solution in PBS of the MTT (3,-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma-Aldrich) tetrazolium substrate was added and incubated for 4 h at 37 ˚C. The resulting violet formazan precipitate was solubilized by the addition of 100 μL of a 50% N,N,-dimethyl formamide/10% SDS solution, and incubated for 4 h at room temperature. Sample absorbances were then measured on a plate reader at 570 nm.
Data were reported as mean ± SD. Statistics were analyzed with the Student’s t-test. A P value of less than 0.05 was considered statistically significant.
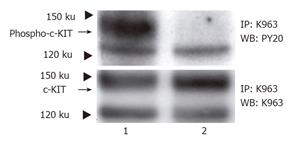
c-KIT in the non-treated cells was tyrosine phosphorylated (Figure 1, upper panel, lane 1) in the GIST-T1 cells. This phosphorylation was inhibited by STI571 treatment (Figure 1, upper panel lane 2). c-KIT protein is also shown by Western blotting (Figure 1, lower panel).
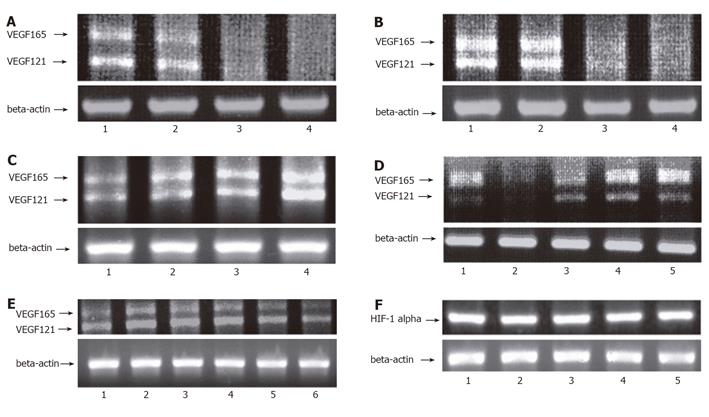
RT-PCR detected the transcription of VEGF or HIF-1 alpha in the GIST-T1 cells. In the GIST-T1 cells, VEGF121 and VEGF165 isoforms were detected (Figures 2A-2D). STI571 inhibited VEGF in a dose- and time-dependent manner (Figures 2A and 2B). On the contrary, STI571 could not inhibit HIF-1 alpha (Figure 2F, lane 2). SCF up-regulated VEGF (Figure 2C) but not HIF-1 alpha (Figure 2F, lane 3). PD98 059, MEK inhibitor, did not inhibit the transcription of VEGF (Figure 2D, lane 4). LY294002 and Wortmannin. both the inhibitors of phosphatidylinositol 3-kinase (PI3K). also did not inhibit the transcription of VEGF in the GIST-T1 cells compared to STI571 (Figure 2D, lanes 3 and 5). SCF (10 ng/mL) upregulated the transcription of VEGF in the colon adenocarcinoma cell line, DLD-1 (Figure 2E, lane 3). However, in the case of high concentration of SCF stimulation (100 ng/mL for 6 h), transcriptions of VEGF were downregulated in the DLD-1 cells (Figure 2E, lane 6).
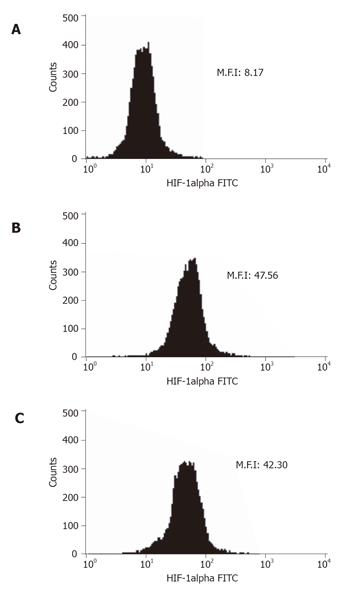
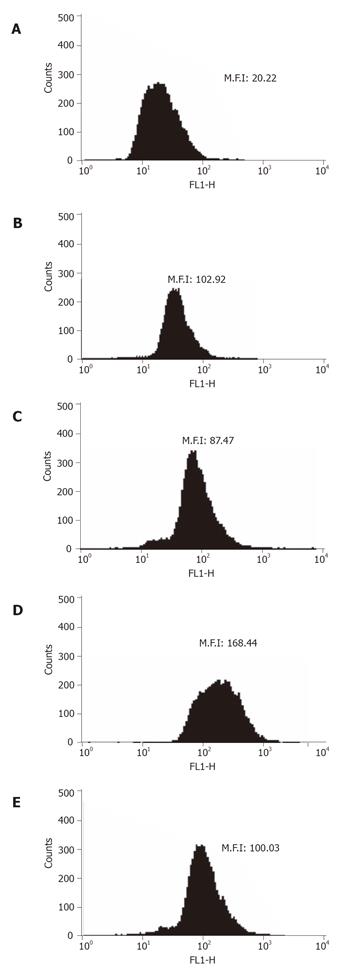
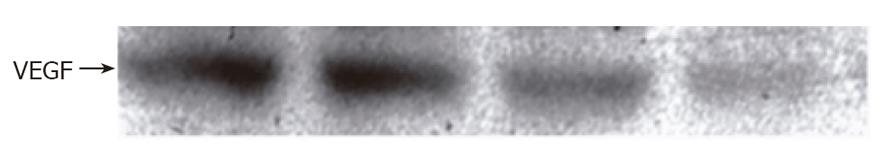
Flow cytometry was used to show that VEGF or HIF-1 alpha was expressed in the non-treated GIST-T1 cells (Figures 3B and 4B). STI571 reduced the expression of VEGF (Figure 4C). SCF upregulated the expression of VEGF in the GIST-T1 cells (Figure 4D) and STI571 inhibited the upregulation of VEGF by SCF stimulation in the GIST-T1 cells (Figure 4E). HIF-1 alpha could not be influenced by STI571 treatment (Figure 3C). SCF also did not influence the expression of HIF-1 alpha (data not shown). Western blotting revealed that VEGF was expressed as a 21-ku band, which was downregulated by ST1571 in a dose-dependent manner (Figure 5).
Cell viability of the GIST-T1 cells was measured by MTT assay, which showed a reduction in viability following the treatment with STI571 to 37.9 ± 1.3%, 36.5 ± 1.7%, and 36.3 ± 0.3% at the concentrations of 0.1, 1, and 2 μg/mL, respectively (P < 0.01 vs non-treated). Cell viability in the case of non-treated cells was indicated as 100 ± 0.5%.
The present study demonstrated that the recently developed compound STI571 inhibited the tyrosine phosphorylation of c-KIT in GIST-T1 tumor cells, as well as inhibiting both the transcription and translation of VEGF. Tyrosine phosphorylation of c-KIT was inhibited after 30 min treatment with STI571 at different concentrations (0.1 and 1 μg/mL), as assessed by Western blot analysis (data not shown). In contrast, Tuveson et al [15] reported that tyrosine phosphorylation of another GIST cell line, GIST882, was not inhibited by a 1-h treatment with STI571 when used at 1 μmol/L (0.59 μg/mL), as assessed by Western blot analysis. GIST882 has a homogenic mutation in the c-kit gene resulting in a K642E amino acid change and the constitutive activation of c-KIT[15]. On the other hand, GIST-T1 has a heterogenic 57-bp deletion in exon 11 of the c-kit mutation, and the c-KIT protein in the GIST-T1 cells constitutively activated. Furthermore, Frolvo et al[16] reported that STI571 inhibits the activation of p44/42 mitogen activated protein kinase (p44/42MAPK) in GIST882 cells, but we could not detect the same effect in our cells following STI571 treatment (data not shown). Oncogenic Ras family proteins activate the p44/42MAPK pathway, and this activation contributes to the increased proliferative rate of tumor cells[17]. Phosphorylated c-KIT also activates the p44/42MAPK pathway as well as plays a role in the process of oncogenic activation[18]. MEK is upstream of p44/42MAPK, and MEK inhibitors could inhibit the activation of p44/42MAPK. In our hands, the MEK inhibitor, PD98059, partially inhibited the activation of p44/42MAPK (data not shown). These results suggested that activation of p44/42MAPK was independent of the activation of c-KIT in GIST-T1 cells.
In a previous study, Ebos et al [19] reported that STI571 reduced VEGF expression in the Bcr-Abl positive chronic myelogenous leukemia cells. They reported that activation of Bcr-Abl played an important role in VEGF expression. In our study, RT-PCR and flow cytometry analysis revealed that both the transcription and translation of VEGF in GIST-T1 cells were suppressed by STI571 treatment. Western blot analysis revealed that STI571 inhibited the expression of VEGF for 12-h incubation. SCF activated c-KIT and upregulated the expression of VEGF. These results suggested that activation of c-KIT was an important role in both the transcription and the translation of VEGF in the GIST-T1 cells. Furthermore we investigated the signal transduction cascade which was involved in the expression of VEGF in the GIST-T1 cells using MEK inhibitor or PI3K inhibitors. In our study, PD98059, LY294002 and Wortmannin had no such effect on these cells. LY294002 and Wortmannin are both inhibitors of PI3K and thereby inhibit activation of the AKT pathway[20]. AKT promotes angiogenesis through eNOS activation[20]. STI571 inhibited the activation of AKT in GIST882 cells[16], but the same result was not obtained in our GIST-T1 cells by Western blotting (data not shown). These results suggested that neither the p44/42MAPK nor PI3K signaling pathways are involved in the regulation of VEGF expression in GIST-T1 cells.
Hypoxia inducible factor-1 (HIF-1) plays an important role in the expression of VEGF in the malignant tumors. HIF-1 activity is primarily regulated by the levels of HIF-1 alpha in the cells[21]. In our study, transcriptional or translational levels of HIF-1 alpha were not regulated by STI571 treatment or SCF stimulation.
c-KIT is expressed in the colon adenocarcinoma cell line, DLD-1[22]. It is clear that c-KIT regulates the expression of VEGF in other cells. So, we observed whether c-KIT was involved in the upregulation of the transcriptional levels of VEGF using SCF in DLD-1 cells. SCF induced VEGF transcription at a concentration of 5–10 ng/mL. These results suggested that the expression of VEGF might be concerned with the activation of c-KIT.
MTT assay revealed that STI571 inhibited the cell viability of GIST-T1 cells. Activation of c-KIT in the GIST-T1 cells played an important role in cell survival signaling.
In conclusion, our results on the treatment of GIST-T1 cells with STI571 suggest that the expression of VEGF in these cells might be regulated via the c-KIT signal transduction cascade. STI571 inhibited not only cell viability but also the expression of VEGF in GIST-T1 cells, and it could be a useful compound for GIST therapy. Further studies are required to investigate the mechanism underlying VEGF regulation in more detail.
We thank Ms Motoko Miyata and Ms Yuka Takezaki for their technical assistance.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3215] [Cited by in F6Publishing: 3008] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 2. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] [Cited in This Article: ] |
| 3. | Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1-96. [Cited in This Article: ] |
| 4. | Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1032] [Cited by in F6Publishing: 1056] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 5. | Longley BJ Jr, Metcalfe DD, Tharp M, Wang X, Tyrrell L, Lu SZ, Heitjan D, Ma Y. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci USA. 1999;96:1609-1614. [Cited in This Article: ] |
| 6. | Tian Q, Frierson HF Jr, Krystal GW, Moskaluk CA. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol. 1999;154:1643–1647. [Cited in This Article: ] |
| 7. | Beghini A, Peterlongo P, Ripamonti CB, Larizza L, Cairoli R, Morra E, Mecucci C. C-kit mutations in core binding factor leukemias. Blood. 2000;95:726-727. [PubMed] [Cited in This Article: ] |
| 8. | Kitamura Y, Hirota S, Nishida T. Gastrointestinal stromal tumors (GIST): a model for molecule-based diagnosis and treatment of solid tumors. Cancer Sci. 2003;94:315-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3203] [Cited by in F6Publishing: 3013] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 10. | Stimpfl M, Tong D, Fasching B, Schuster E, Obermair A, Leodolter S, Zeillinger R. Vascular endothelial growth factor splice variants and their prognostic value in breast and ovarian cancer. Clin Cancer Res. 2002;8:2253-2259. [PubMed] [Cited in This Article: ] |
| 11. | Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003;64:266-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Taguchi T, Sonobe H, Toyonaga S, Yamasaki I, Shuin T, Takano A, Araki K, Akimaru K, Yuri K. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stromal tumor. Lab Invest. 2002;82:663-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Knox AJ, Corbett L, Stocks J, Holland E, Zhu YM, Pang L. Human airway smooth muscle cells secrete vascular endothelial growth factor: up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB J. 2001;15:2480-2488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Nakayama K, Kanzaki A, Hata K, Katabuchi H, Okamura H, Miyazaki K, Fukumoto M, Takebayashi Y. Hypoxia-inducible factor 1 alpha (HIF-1 alpha) gene expression in human ovarian carcinoma. Cancer Lett. 2002;176:215-223. [Cited in This Article: ] |
| 15. | Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD, Fletcher JA, Demetri GD. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054-5058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 535] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 16. | Frolov A, Chahwan S, Ochs M, Arnoletti JP, Pan ZZ, Favorova O, Fletcher J, von Mehren M, Eisenberg B, Godwin AK. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther. 2003;2:699-709. [PubMed] [Cited in This Article: ] |
| 17. | Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911-1912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3062] [Cited by in F6Publishing: 3171] [Article Influence: 144.1] [Reference Citation Analysis (0)] |
| 18. | Frost MJ, Ferrao PT, Hughes TP, Ashman LK. Juxtamembrane mutant V560GKit is more sensitive to Imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol Cancer Ther. 2002;1:1115-1124. [PubMed] [Cited in This Article: ] |
| 19. | Ebos JM, Tran J, Master Z, Dumont D, Melo JV, Buchdunger E, Kerbel RS. Imatinib mesylate (STI-571) reduces Bcr-Abl-mediated vascular endothelial growth factor secretion in chronic myelogenous leukemia. Mol Cancer Res. 2002;1:89-95. [PubMed] [Cited in This Article: ] |
| 20. | Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1407] [Cited by in F6Publishing: 1618] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 21. | Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995-4004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 945] [Cited by in F6Publishing: 938] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 22. | Bellone G, Carbone A, Sibona N, Bosco O, Tibaudi D, Smirne C, Martone T, Gramigni C, Camandona M, Emanuelli G. Aberrant activation of c-kit protects colon carcinoma cells against apoptosis and enhances their invasive potential. Cancer Res. 2001;61:2200-2206. [PubMed] [Cited in This Article: ] |