Published online Dec 28, 2006. doi: 10.3748/wjg.v12.i48.7826
Revised: October 28, 2006
Accepted: November 23, 2006
Published online: December 28, 2006
AIM: To summarize the evidence available for the clinical effectiveness of insulin sensitizers in the treatment of nonalcoholic fatty liver disease (NAFLD) systematically.
METHODS: Relevant articles were located using computer-assisted searches of Medline (1966-March 2006), EMBASE (1988-March 2006), CINAHL (1982-March 2003), Educational Resource Information Center (1966-March 2006), Library, Information Science & Technology Abstracts (1967-March 2006), Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (1994-2006), dissertations in ProQuest and FirstSearch databases. Manual searches were made in the abstracts from meetings of the American Gastroenterological Association (1999-2006), and the American Association for the Study of Liver Diseases (2003-2005). Studies were retrieved using the following selection criteria: (1) clinical trials using insulin sensitizers in subjects with NAFLD, (2) adult patients, (3) published as full manuscripts or abstracts, and (4) English, Spanish, German, and French languages only. Data were abstracted independently by two reviewers following standardized procedures. A face-to-face comparison of data was conducted to ensure the completeness and reliability of the abstraction process.
RESULTS: Nine studies were included, six using metformin and three using thiazolidinediones. Only two studies were placebo-controlled trials. The median sample size for all studies was 18 subjects. In the placebo-controlled trials, metformin improved insulin resistance markers and liver function tests, but not histological scores. In the single-arm trials, metformin and thiazolidinediones improved insulin resistance markers and liver function tests, and beneficial histological changes were reported. There is limited high-quality information available from which to draw categorical conclusions about the clinical use of insulin sensitizers in NAFLD.
CONCLUSION: Current information indicates that the use of insulin sensitizers in NAFLD improves insulin resistance and liver function. Histological changes must be corroborated in randomized controlled trials.
- Citation: Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Ávila FI, Sánchez-Ávila F, Montaño-Reyes MA, Uribe M. Insulin sensitizers in treatment of nonalcoholic fatty liver disease: Systematic review. World J Gastroenterol 2006; 12(48): 7826-7831
- URL: https://www.wjgnet.com/1007-9327/full/v12/i48/7826.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i48.7826
Nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized condition that may progress to end-stage liver disease, ranging from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis (in 1.6% of patients with NAFLD). The pathological picture resembles that of alcohol-induced liver injury, but it occurs in patients who do not abuse alcohol[1]. The true prevalence of NAFLD in the USA is unknown. Based on the percentage of people in the Third National Health and Nutrition Examination Survey (NHANES-III) with unexplained elevated levels of serum aminotransferase, up to 7.3% of the USA population could be suffering from NAFLD[2]. When the diagnostic criteria are modified, the estimated prevalence of NAFLD reaches 24%[3]. According to Byron et al[4], NAFLD is the third most common diagnosis in gastroenterological referrals, accounting for 11% of patients. NAFLD is expected to become one of the most important liver diseases in the near future as a result of the obesity epidemic[5].
NAFLD was first described more than 20 years ago[1] and many advances in our understanding of its pathophysiological mechanisms have been made. Diet and exercise constitute the central strategies in NAFLD treatment[6]. Considering the pathogenic mechanisms that may be involved, several pharmacological strategies for NAFLD have been tested that focus on correcting the risk factors for insulin resistance and decreasing hyperinsulinemia, as hepatoprotective effects, using diverse drugs: gemfibrozil, metformin, betaine, N-acetylcysteine, and vitamin E[7]. However, no consensus regarding an effective therapy for NAFLD has been reached[8].
Over the last five years, clinical trials evaluating the use of insulin sensitizers in the treatment of NAFLD, such as metformin and thiazolidinediones, have been conducted. Mixed results, heterogeneous therapeutic approaches, and the small numbers of subjects have limited their application as clinical guidelines. We performed a comprehensive systematic review to summarize the evidence available for the clinical effectiveness of insulin sensitizers in the treatment of NAFLD.
Relevant articles were located using computer-assisted searches of Medline (1966-March 2006), EMBASE (1988-March 2006), Cumulative Index to Nursing & Allied Health Literature (CINAHL) (1982-March 2003), Educational Resource Information Center (ERIC) (1966-March 2006), Library, Information Science & Technology Abstracts (LISTA) (1967-March 2006), Cochrane Database of Systematic Reviews (CDSR), Cochrane Controlled Trials Register (CCTR), Database of Abstracts of Reviews of Effects (DARE) (1994-2006), dissertations in ProQuest and FirstSearch databases, and Literatura Latinoamericana y del Caribe en Ciencias de la Salud (LILACS). Manual searches were conducted in the abstracts from the Digestive Disease Week of the American Gastroenterological Association (1999-2006), the American Association for the Study of Liver Diseases Meetings (2003-2005), and the reference lists included in the retrieved articles. Searching terms included: nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, hepatic steatosis, treatment, clinical trial, metformin, thiazolidinediones, rosiglitazone, troglitazone, pioglitazone, englitazone, PPAR-gamma, peroxisomal proliferator activated receptor.
Two investigators (ChN, BT) independently reviewed the titles and abstracts of all the citations identified. Potentially relevant studies were retrieved based on the following selection criteria: (1) clinical trials using one or a combination of insulin sensitizers (metformin and thiazolidinediones: rosiglitazone, troglitazone, pioglitazone, englitazone) in subjects with NAFLD, (2) adult patients, (3) data published in full manuscript form or abstract form, and (4) English, Spanish, German, and French languages only.
After retrieval, the articles were subject to evaluation to ensure their compliance with the inclusion and exclusion criteria considered for data abstraction. The exclusion criteria for data abstraction from the selected studies were: (1) use of a concomitant therapeutic approach (ursodeoxycholic acid, antioxidants, etc.) with insulin sensitizers, and (2) less than 10 participants at the beginning of the study. Inclusion criteria were: (1) controlled trials of insulin sensitizers versus placebo or diet, (2) single-arm studies of rosiglitazone, troglitazone, pioglitazone, or englitazone, and (3) NAFLD or nonalcoholic steatohepatitis (NASH) based on histological diagnosis or imaging studies (computed tomography, abdominal ultrasound, or magnetic resonance imaging) and/or aberrant liver function tests in the absence of alcohol consumption. Unblinded evaluation of the inclusion/exclusion criteria was conducted separately by all authors. Discrepancies in selection were resolved by consensus. Checks for repeated references were conducted based on the authors’ names, publication dates, and reported population characteristics. Data abstraction was conducted independently by ChN and BT following the standardized procedures developed by the research team. The criteria included: publication and study characteristics, study population, diagnostic criteria, intervention description, and baseline and postintervention clinical characteristics. In cases where the information to be abstracted was not presented in the published reports, the authors attempted to contact the corresponding authors (Blaszyk, Azuma, and Duseja), and the responses (Duseja) were included in the abstraction formats. After all studies were abstracted, a face-to-face comparison of data retrieved by ChN and BT was conducted to ensure the completeness and reliability of the abstraction process. Minor discrepancies were recorded and resolved by referring to the original paper.
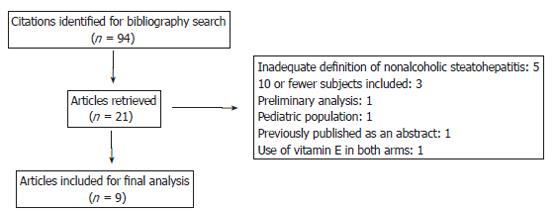
A total of 94 studies were retrieved from the broad search terms used (Figure 1). After elimination of editorials, reviews, and repeated reports, 10 studies[9-18] remained candidates for evaluation. One study was excluded because of concomitant use of vitamin E[18] (Table 1). Heterogeneity of treatments, methodologies, and reporting quality in the studies precluded any attempt to estimate summary measures, forcing a narrative presentation of our findings. All studies were classified into one of three groups based on the characteristics of the pharmacological interventions: metformin vs diet trials, metformin single-arm trials, and thiazolidinedione single-arm trials.
| Ref. Design | Participants | Intervention | Outcomes | ||
| IR | Biochemical | Histological | |||
| [9] Nonrandomized | Italy. 20 consecutive subjects. | Treatment: metformin 500 mg/d for 4 mo | + | + | NE |
| open-label | No diabetic or severely obese subjects. | Control: diet | |||
| controlled | |||||
| [10] Randomized | Turkey. 36 nondiabetic subjects. | Treatment: metformin 1.7 g/d for 6 mo | + | + | – |
| open-label | Control: diet (1600–1800 cal/d) | ||||
| controlled | |||||
| [11] Nonrandomized | USA. 15 subjects. | Metformin 20 mg/kg per day for 48 wk | + at 3 mo | + at 3 mo | +2 |
| open-label | One diabetic. | + at end | –(+ ) at end | ||
| single-arm trial | of study | of study | |||
| [12] Nonrandomized | USA. 10 subjects. | Metformin 2 g/d for 48 wk | - | - | - |
| open-label | |||||
| single-arm trial | |||||
| [13] Randomized controlled trial1 | Italy. 17 subjects. | Metformin 2 g/d for 48 wk | + | + | + |
| [14] Nonrandomized | India. 22 subjects. | Metformin 1.5 g/d for 6 mo | + | + | NE |
| open-label | Three diabetics. | ||||
| single-arm trial | |||||
| [15] Nonrandomized | USA. 25 subjects. | Rosiglitazone 8 mg/d for | + | + | + |
| open-label | 48 wk plus diet and physical activity | ||||
| single-arm trial | |||||
| [16] Nonrandomized | USA. 18 nondiabetic subjects. | Pioglitazone 30 mg/d for 48 wk | + | + | + |
| open-label | |||||
| single-arm trial | |||||
| [17] Nonrandomized | Japan. 12 subjects. | Pioglitazone 30 mg/d for 12 wk | + | + | NE |
| open-label | |||||
| single-arm trial | |||||
Most (77%) studies were designed as single-arm trials, one was designed as a randomized controlled trial, and one as a nonrandomized controlled trial. Sample sizes ranged from 10 to 36 subjects, with a median of 18 subjects. All studies used a histological diagnosis of NASH as the inclusion criterion, and posttreatment biopsies were available in only six studies.
Metformin versus diet trials: Two studies compared the efficacy of metformin versus diet in the treatment of NAFLD. Marchesini et al[9] studied 20 consecutive patients (no diabetic or severely obese subjects were included) with liver function tests, and tests for insulin and insulin resistance (by euglycemia and a hyperinsulinemic glucose clamp). Liver biopsies were conducted in 14 subjects who received metformin (500 mg tid) and six were treated with diet alone for four months. The only significant difference between the two groups was in their alanine aminotransferase values. Histological improvement was not evaluated. The diet group did not differ from the drug group in weight reduction, which could reflect the effect of metformin. The most common adverse effect was gastrointestinal. Although subjects undergoing active treatment showed increased levels of lactic acid (by 30% in actively treated patients), just one patient was above the normal range of 2 mmol/L (2.2 mmol/L).
Uygun et al[10] studied 36 patients with NAFLD. The treatment group received metformin (850 mg bid) plus dietary treatment. The control group received only a restricted diet (1600-1800 calories per day). Compared with the controls, the treatment group showed improvements in: alanine aminotransferase (37.1 vs 17.4 U/L, respectively, P = 0.003), aspartate aminotransferase (22.1 vs 6.8 U/L, respectively, P = 0.0001), body mass index (2.4 vs 1.9 kg/m2, respectively, P = 0.01), and index of insulin resistance (1.15% vs 0.02%, respectively, P = 0.001). In fact, a comparison of the treatment group at baseline and at six months showed improvements in alanine aminotransferase (83.5 ± 24.6 vs 46.4 ± 23.3 U/L, respectively, P = 0.0001) and aspartate aminotransferase (57.9 ± 17.3 vs 35.8 ± 10.5 U/L, respectively, P = 0.0001). However, this was also seen in the control group: alanine aminotransferase (72.8 ± 31.2 vs 55.4 ± 16.3 U/L, respectively, P = 0.001) and aspartate aminotransferase (48.1 ± 26.3 vs 41.3 ± 13.5 U/L, respectively, P = 0.06). No differences were observed in the liver biopsies of subjects after treatment. No patient discontinued metformin because of a lack of tolerance for the treatment. No patient reported symptoms of hypoglycemia. Four patients complained of gas and bloating and two patients complained of mild to moderate abdominal pain in the first month. However, these complaints did not require cessation of the drug.
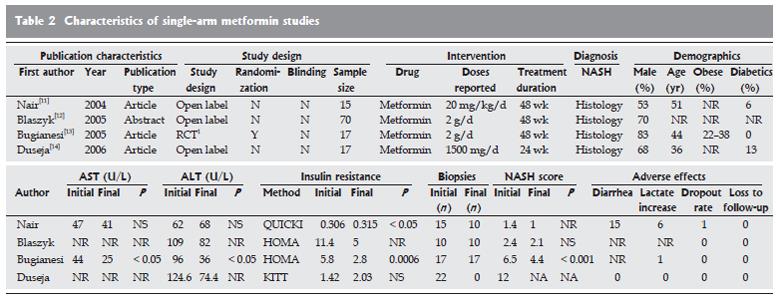
Metformin single-arm trials: Four single-arm trials evaluated the use of metformin in NAFLD (Figure 2)[11-14]. The mean age of the study participants ranged from 36 to 51 years in all but one study[14]. Males were predominant (ranging from 53% to 83%). The doses used in the different series ranged from 20 mg/kg per day (approximately 1.4 g per day in a subject of 70 kg) to 2 g/d. Treatment duration varied from six months[14] to 48 wk. Insulin resistance was assessed by the QUICKI, HOMA, or KITT methods.
All trials reported an improvement in the indices of insulin resistance, three studies[12-14] reported a reduction in liver function test values, and one study reported a nonsignificant increase in these values[11].
In terms of histological improvement, only one report[13] showed statistical differences in inflammation, steatosis, fibrosis, and global evaluations of NASH after treatment. The most common adverse effects were associated with poor gastrointestinal tolerance. One patient had an increase in serum lactate levels that required the patient to withdraw from the study.
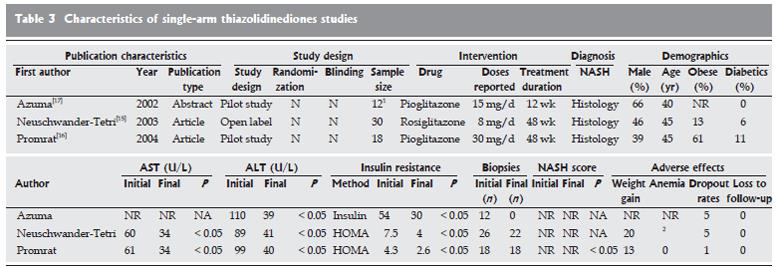
Thiazolidinediones single-arm trials: The use of thiazolidinediones (pioglitazone, rosiglitazone, and troglitazone) was evaluated in three studies (Figure 3)[15-17]. The mean age in each study was 40-46 years. In one study, men were in the majority[17], and in two studies, diabetic subjects were included[15,16]. Of these studies, two used pioglitazone and one used rosiglitazone, at varying doses (pioglitazone 15-30 mg/d and rosiglitazone 4 mg, bid). The durations of the studies ranged from 12 wk[17] (pioglitazone 15 mg/d) to 48 wk. Two studies assessed insulin resistance with HOMA-IR[15,16] and the other with serum insulin levels[17]. Posttreatment hepatic biopsies were reported in two studies[15,16].
All studies showed significant improvement in insulin resistance. Alanine aminotransferase and aspartate aminotransferase showed significant decreases in all studies. Posttreatment biopsies showed statistically significant improvements with respect to baseline biopsies[15,16]. The adverse effects reported were weight gain, serum lactate increases, bad dreams, and heavy legs. The pooled dropout rate was high, at 11 of 60 subjects. No cases of liver failure were reported.
This systematic review analyzes the clinical use of insulin sensitizers in the treatment of NAFLD. Although it has been more than 20 years since the first description of NAFLD[1], and much progress has been made in understanding its epidemiology and pathophysiology, few advances have been made in its treatment.
In this review, only two clinical studies compared pharmacological treatments with diet treatments. The methodological limitations are clear: the small numbers of subjects, nonrandomization and the lack of blinded measures, and the limited use of histological outcomes.
The fact that limited high-quality information available is interesting, especially because (1) NAFLD is a very common disease, with incidences between 3% in the low-risk population[19] and 93% in high-risk subjects[20,21], (2) subjects have some degree of histologically evident chronic liver damage, and at least 30% have fibrosis at diagnosis[22], (3) it is an important cause of chronic liver failure and adversely affects survival rates, with 7-10-year liver-related mortality rates of 12% to 25%[23], and (4) it is an important factor in cardiovascular-related mortality; in a 10-year prospective study of subjects with NASH or hepatitis C viral infection, the mortality rates were 5.2% vs 0.6%, respectively (P < 0.03)[24].
Analyzing the usefulness of insulin sensitizers by comparing metformin with thiazolidinediones in single-arm trials suggests that thiazolidinediones are the better option. However, when diet-controlled studies are considered, this conclusion is less clear because, contrary to the single-arm trials, these studies indicate that the use of metformin clearly benefits liver enzymes. Unfortunately, no data from a well-designed head-to-head comparative clinical trial are available to answer this question. In all the studies analyzed, a heterogeneity of drugs and doses was observed, which made it more difficult to evaluate the efficacy of insulin sensitizers in clinical practice.
The evidence presented in this systematic review indicates that the treatment of NAFLD with insulin sensitizers has been, until now, a nebulous field. However, new well-designed trials have been in progress during the preparation of this paper. Four trials using metformin and three studies on thiazolidinediones are in the recruitment phase[25]. Information derived from these studies should help in the clinical management of this disease.
Despite this (and future) information, many issues are not answered: (1) cost-analysis comparing diet and exercise with pharmacological treatment, (2) safety of insulin sensitizers in large samples, and perhaps one of the most important questions that (3) insulin sensitizers only treat one face of the metabolic syndrome and pharmacological approaches to treat all components of the metabolic syndrome sounds too simplistic[26]. This indicates that more creative prevention policy is mandatory.
In conclusion, current information indicates that the use of insulin sensitizers in the treatment of NAFLD improves insulin resistance and liver function. Single-arm studies have shown positive histological changes. However, placebo-controlled trials do not support this histological response. Future information derived from well-designed running trials will be useful in defining the clinical implications of insulin sensitizers in the treatment of NAFLD.
S- Editor Liu Y L- Editor Zhu LH E- Editor Bai SH
| 1. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] [Cited in This Article: ] |
| 2. | Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 227] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 3. | Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 630] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 4. | Byron D, Minuk GY. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology. 1996;24:813-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Méndez-Sánchez N, Villa AR, Chávez-Tapia NC, Ponciano-Rodriguez G, Almeda-Valdés P, González D, Uribe M. Trends in liver disease prevalence in Mexico from 2005 to 2050 through mortality data. Ann Hepatol. 2005;4:52-55. [PubMed] [Cited in This Article: ] |
| 6. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1488] [Cited by in F6Publishing: 1434] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 7. | Méndez-Sánchez N, Chávez-Tapia NC, Uribe M. [An update on non-alcoholic fatty liver disease]. Rev Invest Clin. 2004;56:72-82. [PubMed] [Cited in This Article: ] |
| 8. | American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 469] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Blaszyk H, Ferrentino N, Forsell S, Strader D, Lidofsky S. A Pilot Study of Metformin As Treatment for Nonalcoholic Steatohepatitis. Gastroenterology. 2005;122:M1699. [Cited in This Article: ] |
| 13. | Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 511] [Cited by in F6Publishing: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 14. | Duseja A, Das R, Das A, Dhiman RK, Chawla YK, Garewal G. Serum iron levels and hepatic iron overload in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2006;51:1730-1731. [PubMed] [Cited in This Article: ] |
| 15. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008-1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 372] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 575] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 17. | Azuma T, Tomita K, Kato S, Adachi H, Inokuchi S, Kitamura N, Nishimura T, Ishii H. A pilot study of a thiazolidinedione, pioglitazone, in nonalcoholic steatohepatitis. Hepatology. 2002;28:406A. [Cited in This Article: ] |
| 18. | Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 431] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Marceau P, Biron S, Hould FS, Marceau S, Simard S, Thung SN, Kral JG. Liver pathology and the metabolic syndrome X in severe obesity. J Clin Endocrinol Metab. 1999;84:1513-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 287] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Luyckx FH, Desaive C, Thiry A, Dewé W, Scheen AJ, Gielen JE, Lefèbvre PJ. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Amarapurka DN, Amarapurkar AD, Patel ND, Agal S, Baigal R, Gupte P, Pramanik S. Nonalcoholic steatohepatitis (NASH) with diabetes: predictors of liver fibrosis. Ann Hepatol. 2006;5:30-33. [PubMed] [Cited in This Article: ] |
| 23. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1756] [Cited by in F6Publishing: 1769] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 24. | Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Heuman D, Coterrell A, Fisher RA. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 341] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 25. | Available from: http://www.clinicaltrials.gov/. Access: 1st april. [Cited in This Article: ] |
| 26. | Amarenco P. Polypill strategy vs. prevention clinics for stroke prevention. Cerebrovasc Dis. 2006;21 Suppl 1:35-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |