Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7666
Revised: November 10, 2006
Accepted: November 20, 2006
Published online: December 21, 2006
AIM: To examine the effects of anti-high mobility group box 1 (HMGB1) neutralizing antibody in experimental severe acute pancreatitis (SAP).
METHODS: SAP was induced by creating closed duodenal loop in C3H/HeN mice. SAP was induced immediately after intraperitoneal injection of anti-HMGB1 neutralizing antibody (200 μg). Severity of pancreatitis, organ injury (liver, kidney and lung), and bacterial translocation to pancreas was examined 12 h after induction of SAP.
RESULTS: Anti-HMGB1 neutralizing antibody significantly improved the elevation of the serum amylase level and the histological alterations of pancreas and lung in SAP. Anti-HMGB1 antibody also significantly ameliorated the elevations of serum alanine aminotransferase and creatinine in SAP. However, anti-HMGB1 antibody worsened the bacterial translocation to pancreas.
CONCLUSION: Blockade of HMGB1 attenuated the development of SAP and associated organ dysfunction, suggesting that HMGB1 may act as a key mediator for inflammatory response and organ injury in SAP.
- Citation: Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol 2006; 12(47): 7666-7670
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7666
In severe acute pancreatitis (SAP), multiple organ dysfunction syndrome (MODS) in the early phase[1,2] and complications of infection (infected pancreatic necrosis and sepsis) in the late phase are contributors to high mortality in SAP[3,4]. MODS is a consequence of the systemic inflammatory response syndrome, and it is conceivable that release of humoral mediators from the excessive activated macrophages/monocytes and neutrophils may lead to the remote organ injury. Complications of infection are thought to be a result of bacterial translocation from the gastrointestinal tract, and breakdown of intestinal integrity is considered to be implicated in the mechanism[5-7].
High mobility group box 1 (HMGB1) protein, originally discovered 30 years ago as a nuclear DNA binding protein[8-10], was recently identified as a late-acting mediator of endotoxin lethality[11]. Injection of HMGB1 itself was lethal, and serum levels of HMGB1 increased from 8 to 32 h after the administration of endotoxin, when the tumor necrosis factor (TNF) peak had already occurred[11]. Antibodies to HMGB1 attenuated the mortality associated with endotoxemia, even when the antibodies were administered 2 h after the onset of endotoxemia[11]. HMGB1 was also found to have the capacity to induce cytokines and activate inflammatory cells when it was applied extracellularly[11-13]. This implicates HMGB1 as a proinflammatory mediator. Recent investigations reported that serum HMGB1 levels increased in patients with sepsis/endotoxemia[11,14,15], hemorrhagic shock[16], acute lung injury[17,18], rheumatoid arthritis[19] and disseminated intravascular coagulation[20]. It has been demonstrated that HMGB1 is secreted actively by living inflammatory cells such as stimulated macrophages/monocytes, and is released passively from necrotic or damaged cells[21-23]. Therefore, HMGB1 may be related to inflammation and necrosis in SAP, and may be an important mediator for multiple organ failure.
In a recent study, we have first demonstrated that serum HMGB1 levels were significantly elevated in patients with SAP on admission, and were correlated with the severity[24,25] of the disease. The HMGB1 levels were higher in patients with organ dysfunction and infection during the clinical course. The HMGB1 levels in non-survivors were higher than those in survivors. These results suggest that HMGB1 may play a pivotal role in the pathogenesis of SAP, and that HMGB1 may act as a key mediator for inflammation and organ failure in this disease. In the present study, to clarify the role of HMGB1 in the pathophysiology of SAP, effects of anti-HMGB1 neutralizing antibody were investigated in SAP in mice.
Female C3H/HeN mice (weighing 20-22 g, 9 weeks old) were purchased from CLEA Japan (Tokyo, Japan). The protocol for this animal experiment was approved by the Institutional Animal Committee of Kobe University Graduate School of Medical Sciences.
Anti-HMGB1 neutralizing antibody (chicken anti-HMGB1 polyclonal antibody) was obtained from Shino-Test Corporation (Sagamihara, Japan). This antibody recognizes mouse HMGB1. The specificity and neutralizing activity of this antibody was confirmed by western blot analysis.
Under general anesthesia with a subcutaneous injection of carbamic acid ethyl ester (urethane) at a dosage of 1.5 g/kg, a midline laparotomy was performed, and a closed loop (2 cm in length) was created by ligating the duodenum at 1 cm proximal and distal sides to the biliopancreatic duct outlet. Only laparotomy was performed in sham-operated mice.
Saline (0.2 mL) or anti-HMGB1 neutralizing antibody (200 μg, 0.2 mL) was injected intraperitoneally, and immediately closed duodenal loop-induced pancreatitis was made. Mice were divided into three groups as follows. Group A: Sham, laparotomy with saline injection. Group B: SAP, severe acute pancreatitis with saline injection. Group C: HMGB1 Ab + SAP, severe acute pancreatitis with anti-HMGB1 antibody injection. Mice were sacrificed 12 h after induction of SAP. Pancreas and lung tissue was removed, fixed in 10% formalin, and stained with hematoxylin and eosin for light microscopic analysis. Blind analysis was carried out for all histological studies. Blood sample was drawn from heart. Serum amylase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), and creatinine (Cr) levels were measured using standard clinical automated analyzer.
The skin was cleaned with 10% povidone iodine. Pancreas was obtained 0, 4, 8, and 12 h after induction of SAP under sterile conditions, and processed for culture of aerobic and anaerobic organisms using a standardized method. Specimens were inoculated onto agar plates including BTB agar, sheep blood agar, chocolate agar (Nippon Becton Dickinson Co. Ltd., Tokyo, Japan), brucella HK agar (Kyokuto Pharmaceutical Co. Ltd., Tokyo, Japan), and GAM (Gifu Anaerobic Medium) agar (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan). BTB agar was incubated in the aerobic chamber at 37°C, sheep blood agar and chocolate agar were incubated in the O2/CO2 incubator, and brucella HK agar was incubated in the anaerobic chamber for 48 h, respectively. GAM agar was incubated in the ambient chamber at 37°C for 72 h. When the colony forming was detected, it was considered to be positive for bacterial translocation.
The results are expressed as mean ± SE. The Mann-Whitney U test and Chi-square test were used to evaluate differences between two groups. A P value < 0.05 was considered statistically significant.
Twelve hours after induction of SAP, serum amylase levels were significantly elevated in SAP group, and anti-HMGB1 neutralizing antibody significantly reduced its elevation (Table 1).
| Parameter | Sham(n = 6) | SAP(n = 20) | HMGB1 Ab + SAP(n = 12) |
| Amylase (IU/L) | 2220 ± 707 | 52 155 ± 14 449a | 8994 ± 1623c |
| AST (IU/L) | 1499 ± 335 | 5056 ± 545a | 4193 ± 561 |
| ALT (IU/L) | 395 ± 288 | 1215 ± 118a | 848 ± 145c |
| LDH (IU/L) | 6743 ± 1206 | 16403 ± 1072a | 15 220 ± 4687 |
| BUN (mg/dL) | 32 ± 3 | 93 ± 6a | 94 ± 7 |
| Cr (mg/dL) | 0.10 ± 0.00 | 0.42 ± 0.10a | 0.16 ± 0.03c |
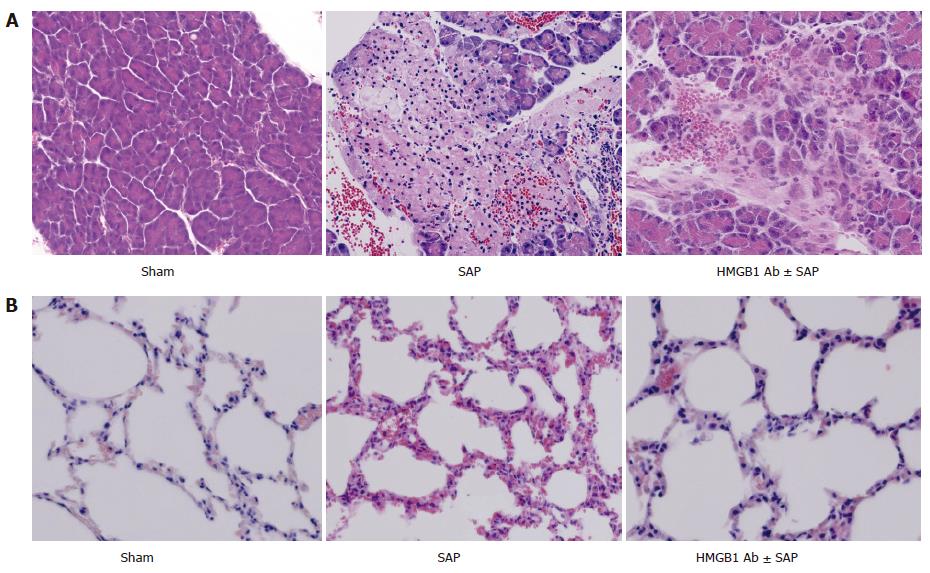
Twelve hours after induction of SAP, HE staining of the pancreas showed edema, hemorrhage, leukocyte infiltration, and necrosis. Anti-HMGB1 neutralizing antibody improved the histological alterations of pancreas (Figure 1A). Twelve hours after induction of SAP, HE staining of the lung showed edema, inflammatory infiltration, hemorrhage and thickening of the alveolar membrane. In contrast, anti-HMGB1 neutralizing antibody ameliorated the histological changes in the lungs (Figure 1B).
Twelve hours after induction of SAP, serum AST, ALT, LDH, BUN, and Cr levels were significantly elevated in SAP group, and anti-HMGB1 neutralizing antibody significantly improved the elevated ALT and Cr (Table 1).
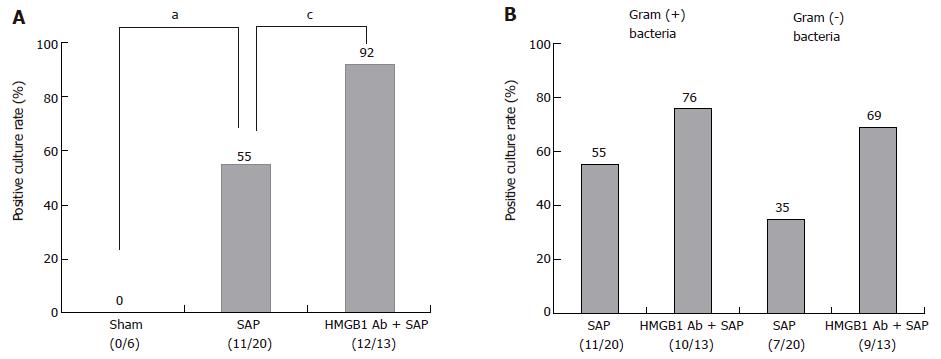
Bacterial translocation to pancreas was not observed in Sham group (Figure 2A), but could be detected 12 h after the induction of SAP. In earlier periods (0, 4, and 8 h), it was not detected. In SAP group, 55% of mice (11/20) exhibited positive bacterial culture. Anti-HMGB1 antibody significantly increased the positive culture rate to 92% (12/13) (Figure 2A). Positive rate of gram-positive and gram-negative bacterial culture in SAP group was 55% (11/20) and 35% (7/20), respectively. Anti-HMGB1 antibody increased them to 76% (10/13) and 69% (9/13), respectively, but no significant difference was observed (Figure 2B).
Extracellular HMGB1 was recently identified as a novel proinflammatory cytokine. In a previous study, we demonstrated that serum HMGB1 levels were significantly elevated in patients with SAP, and were correlated with disease severity[24]. In this study, we have for the first time demonstrated that blockade of HMGB1 attenuated the development of SAP and associated organ dysfunction, suggesting that HMGB1 may act as a key mediator for inflammatory response and organ injury in SAP. We think that raised HMGB1 may represent a cause of aggravation of SAP (progression to SAP) and associated organ dysfunction as well as a consequence of SAP. On the other hand, HMGB1 can promote alterations in gut barrier function by increasing the permeability in enterocytic monolayers and increasing bacterial translocation in mice[26]. Similar contributions of HMGB1 to SAP were supposed, but blockade of HMGB1 adversely worsened the bacterial translocation against our expectation.
There have been several reports concerning effects of anti-HMGB1 neutralizing antibody in other pathological conditions. It has been demonstrated that anti-HMGB1 antibody protected against organ injury and improved survival in murine sepsis[14] and rat sepsis[27]. Tsung et al[28] clarified that inhibition of HMGB1 with neutralizing antibody significantly decreased liver damage after ischemia/reperfusion, whereas administration of recombinant HMGB1 worsened it. It has been reported that anti-HMGB1 antibody improved lipopolysaccharide (LPS)-induced acute lung injury in mice[18], and ventilator-induced lung injury in rabbits[29]. These observations together with our results in this study indicate that HMGB1 is one of the deteriorating factors in the development of organ injury.
Concerning the elevation of serum HMGB1 levels in SAP, two possible mechanisms can be assumed[24]. First, HMGB1 may be produced and released by macrophages/monocytes in response to inflammatory mediators. In SAP, it is conceivable that release of humoral mediators from the excessive activated macrophages/monocytes may lead to the remote organ injury. Thus, release of HMGB1 from activated macrophages/monocytes may participate in tissue injury and organ failure in SAP. Second, HMGB1 may be produced and released by injured pancreas or other damaged organs. Recent investigations demonstrated that HMGB1 mRNA expression was significantly increased in liver and lung after rat thermal injury[30], that HMGB1 concentration was increased in lung epithelial lining fluid of patients with acute lung injury[18], and that HMGB1 expression was up-regulated in the liver after murine liver ischemia-reperfusion[28]. Therefore, it is likely that HMGB1 is produced and released by damaged organs in SAP. Change of HMGB1 expression in various tissues should be investigated in SAP.
It was recently clarified that extracellular HMGB1 acts as a cytokine by signaling via the receptor for advanced glycated end-products (RAGE)[31,32] and/or via members of the toll-like receptor (TLR) family (TLR2 and 4)[33]. Activation of RAGE and TLR leads to the induction of inflammatory responses via NF-κB. Tsung et al[28] demonstrated that anti-HMGB1 antibody failed to provide protection in TLR4-defective mice, but successfully reduced liver damage after ischemia/reperfusion in wild-type mice, suggesting that TLR4 is involved in the process as one of the receptors. As TLR4 recognizes LPS of gram-negative bacilli[34,35], interactions of HMGB1 with TLR4 may provide an explanation for the ability of HMGB1 to generate inflammatory responses that are similar to those initiated by LPS. Moreover, TLR is involved in not only inflammatory response but also host defense mechanism, and it is postulated that TLR may function defensively against infection. In our result, anti-HMGB1 antibody worsened the bacterial translocation (especially gram-negative bacteria) in SAP, suggesting that HMGB1 may function at least partially via TLR (especially TLR4).
Results obtained here raise the possibility that blockade of HMGB1 in the early phase is useful as a new therapeutic option against the inflammatory response and MODS in patients with SAP. Further investigations should be performed to elucidate the role of HMGB1 and HMGB1 signaling in the mechanism of inflammatory response, organ injury, and infection in SAP.
S- Editor Wang GP L- Editor Ma JY E- Editor Bi L
| 1. | Tenner S, Sica G, Hughes M, Noordhoek E, Feng S, Zinner M, Banks PA. Relationship of necrosis to organ failure in severe acute pancreatitis. Gastroenterology. 1997;113:899-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 396] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433-438. [PubMed] [Cited in This Article: ] |
| 4. | Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 565] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 5. | Runkel NS, Moody FG, Smith GS, Rodriguez LF, LaRocco MT, Miller TA. The role of the gut in the development of sepsis in acute pancreatitis. J Surg Res. 1991;51:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Medich DS, Lee TK, Melhem MF, Rowe MI, Schraut WH, Lee KK. Pathogenesis of pancreatic sepsis. Am J Surg. 1993;165:46-50; discussion 51-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Gianotti L, Munda R, Alexander JW, Tchervenkov JI, Babcock GF. Bacterial translocation: a potential source for infection in acute pancreatitis. Pancreas. 1993;8:551-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 567] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Melvin VS, Edwards DP. Coregulatory proteins in steroid hormone receptor action: the role of chromatin high mobility group proteins HMG-1 and -2. Steroids. 1999;64:576-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237-5246. [PubMed] [Cited in This Article: ] |
| 11. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2606] [Cited by in F6Publishing: 2624] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 12. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1100] [Cited by in F6Publishing: 1146] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 13. | O'Connor KA, Hansen MK, Rachal Pugh C, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, Tracey KJ. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 891] [Cited by in F6Publishing: 900] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 15. | Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 340] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Ombrellino M, Wang H, Ajemian MS, Talhouk A, Scher LA, Friedman SG, Tracey KJ. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet. 1999;354:1446-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950-2954. [PubMed] [Cited in This Article: ] |
| 18. | Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 375] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 20. | Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S, Maruyama I. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94:975-979. [PubMed] [Cited in This Article: ] |
| 21. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3079] [Cited by in F6Publishing: 3151] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 22. | Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 302] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Yang H, Tracey KJ. High mobility group box 1 (HMGB1). Crit Care Med. 2005;33:S472-S474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Yamada S, Inoue K, Yakabe K, Imaizumi H, Maruyama I. High mobility group protein 1 (HMGB1) quantified by ELISA with a monoclonal antibody that does not cross-react with HMGB2. Clin Chem. 2003;49:1535-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Ebina M, Yamada S, Hashimoto S, Fukata S, Abraham E. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg. 2006;30:1755-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 903] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 29. | Ogawa EN, Ishizaka A, Tasaka S, Koh H, Ueno H, Amaya F, Ebina M, Yamada S, Funakoshi Y, Soejima J. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Fang WH, Yao YM, Shi ZG, Yu Y, Wu Y, Lu LR, Sheng ZY. The significance of changes in high mobility group-1 protein mRNA expression in rats after thermal injury. Shock. 2002;17:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752-25761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 855] [Cited by in F6Publishing: 883] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 32. | Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Gröne HJ, Kurschus FC, Schmidt AM. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 392] [Cited by in F6Publishing: 388] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 33. | Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370-7377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1174] [Cited by in F6Publishing: 1218] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 34. | Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3900] [Cited by in F6Publishing: 3726] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 35. | Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085-2088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5767] [Cited by in F6Publishing: 5635] [Article Influence: 216.7] [Reference Citation Analysis (0)] |