Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7591
Revised: November 15, 2006
Accepted: November 20, 2006
Published online: December 21, 2006
AIM: To evaluate whether folate levels in mucosal tissue and some common methylenetetrahydrofolate reductase (MTHFR) variants are associated with the risk of gastric cancer through DNA methylation.
METHODS: Real-time PCR was used to study the expression of tumor related genes in 76 mucosal tissue samples from 38 patients with gastric cancer. Samples from the gastroscopic biopsy tissues of 34 patients with chronic superficial gastritis (CSG) were used as controls. Folate concentrations in these tissues were detected by the FOL ACS: 180 automated chemiluminescence system. MTHFR polymorphisms were analyzed by PCR-RFLP, and the promoter methylation of tumor-related genes was determined by methylation-specific PCR (MSP).
RESULTS: Folate concentrations were significantly higher in CSG than in cancerous tissues. Decreased expression and methylation of c-myc accompanied higher folate concentrations. Promoter hypermethylation and loss of p16INK4A in samples with MTHFR 677CC were more frequent than in samples with the 677TT or 677CT genotype. And the promoter hypermethylation and loss of p21WAF1 in samples with MTHFR 677CT were more frequent than when 677CC or 677TT was present. The 677CT genotype showed a non-significant higher risk for gastric cancer as compared with the 677CC genotype.
CONCLUSION: Lower folate levels in gastric mucosal tissue may confer a higher risk of gastric carcinogenesis through hypomethylation and overexpression of c-myc.
- Citation: Weng YR, Sun DF, Fang JY, Gu WQ, Zhu HY. Folate levels in mucosal tissue but not methylenetetrahydrofolate reductase polymorphisms are associated with gastric carcinogenesis. World J Gastroenterol 2006; 12(47): 7591-7597
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7591.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7591
Methylation of gene regulatory elements is a well-documented epigenetic change that can lead to gene inactivation. Human gastric carcinogenesis is suggested to be associated with the decrease of total genomic DNA methylation, hypomethylation of certain specific oncogenes such as c-myc, and hypermethylation of promoter of some tumor suppressor genes containing p16INK4A and hMLH1 gene[1]. Folate (or folic acid) is essential for normal DNA methylation and synthesis. We have performed a series of studies to investigate the interrelationship between DNA methylation and folate status in plasma of patients with gastric cancer[2,3]. The plasma folic acid concentration in patients who showed hypomethylation of c-myc was lower than that in patients showing normal methylation. Low plasma levels of folate have been associated with an increased risk for gastric cancers[4,5].
Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in folate metabolism that regulates the intracellular folate pool. Two MTHFR polymorphisms, C677T and A1298C, are known to be risk factors for gastric cancer in Chinese[6], but not in Korean[7]. The MTHFR 677T allele was significantly associated with gastric cancer risk with an odds ratio (OR) of 2.49 [95% confidence interval (CI): 1.48-4.20] in heterozygous MTHFR 677CT carriers and an OR of 2.85 (95% CI: 1.52-5.35) in homozygous MTHFR 677TT carriers in a high risk Italian population[8]. These findings suggest that common variants of MTHFR may play a role in the etiology of gastric cancer, particularly gastric cardia adenocarcinoma. Future studies using large sample sizes and incorporated detailed data on dietary folate intake and related serological measurements are needed to confirm these findings[9].
The extent to which tissue folate levels and MTHFR 677 (C→T) polymorphism interact to affect DNA methylation in gastric carcinogenesis is uncertain. It is even not clear that there is a relationship between folate concentrations and DNA methylation in gastric mucosal tissue. In the current study, we hypothesized that folate levels and some common MTHFR variants are associated with the risk of gastric cancer through DNA methylation. Our data show that decreased folate in tissues is associated with a higher risk of gastric cancer. However, MTHFR gene polymorphisms are not independent risk factors for initiation and progression of gastric cancer, although the 677CT genotype shows a non-significant higher risk for gastric cancer as compared with the 677CC genotype.
Thirty-eight consecutive patients with gastric cancer underwent resection at Shanghai Renji Hospital between May and December 2004. Clinicopathological factors, tumor histologies and disease stages were evaluated according to the General Rules for Clinical and Pathological Studies on gastric cancer. Paired samples (76) of histologically verified primary gastric cancer and corresponding non-cancerous gastric mucosa (> 5 cm away from cancerous margin) of 38 patients were obtained immediately after surgical resection. HE-stained sections were examined for pathological diagnoses, and were categorized according to the WHO histological classifications of gastric cancer. The histological characteristics of non-cancerous tissues were chronic atrophic gastritis, intestinal metaplasia, or dysplasia. All of tumors were located in gastric antrum or corpus, and not in fundus or cardia. There were 23 cases of tubular adenocarcinoma, 4 cases of mucinous adenocarcinoma and 11 cases of tubular-papillary adenocarcinoma. The mean age of the patients at resection was 61 (range 31-81) years and it included 25 men and 13 women. A portion of each tissue (approximately 3-5 g) was snap-frozen on dry ice and kept in liquid nitrogen until use for DNA or RNA extraction. Another 34 patients with chronic superficial gastritis (CSG) were studied as sex, age and H pylori infection (by histology, urease test or breath test, as well as alcohol and tobacco intake matched controls to the gastric cancer group). Three endoscopic biopsy tissue samples were obtained from each control. All controls were subjected to clinical assessment, upper gastrointestinal endoscopy, histopathology of antral mucosa. No chronic atrophic gastritis, intestinal metaplasia or dysplasia was detected in any of the controls. Complete written consent was obtained from all patients and controls.
One milliliter of PBS was added to 10 mg of mucosal tissues. Lysates were sonicated, and the debris was removed from samples by centrifugation for 10 min at 15 000 ×g, 4°C in a microcentrifuge. Folate levels in gastric mucosa were measured with an ACS: 180 automated chemiluminescence analyzer (Chiron Diagnostics Corporation, East Walpole, MA). The ACS: 180 Folate assay is a direct chemiluminescence competitive immunoassay. Folates in the patient sample competed with acridinium ester-labeled folates in the Lite Reagent for a limited amount of biotin-labeled folate binding proteins. Biotin-labeled folate binding proteins bind to avidin that is covalently coupled to paramagnetic particles in the Solid Phase. The sample was pretreated to release the folates from endogenous binding proteins.
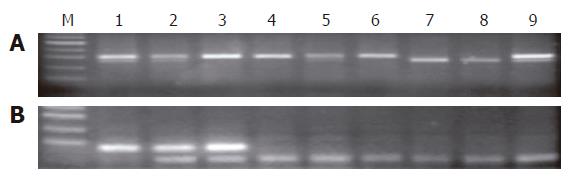
Genomic DNA was isolated from gastric mucosal tissue using QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. MTHFR C677T and A1298C mutations were detected after PCR amplification with the corresponding primers. The restriction enzyme HinfI(New England Biolabs, Beverly, MA) was used to distinguish the 677 (C→T) polymorphism. The variant allele (677TT) gained an HinfIrestriction site and resulted in two fragments of 175 bp and 23 bp after digestion. The wild-type allele (677CC) had a single band representing the entire 198 bp fragment (Figure 1A). The restriction enzyme MboII(New England Biolabs) was used to distinguish the 1298 (A→C) polymorphism. The restriction site was absent in the variant allele (1298CC), and digestion yielded four fragments of 84, 31, 30 and 18 bp, whereas the wild-type allele (1298AA) generated 56, 31, 30, 28 and 18 bp bands (Figure 1B). The restriction products were analyzed by electrophoresis in a 3% agarose gel stained with ethidium bromide. PCR amplifications were run for 35 cycles, with each amplification cycle consisting of 30 s at 94°C, 30 s at 62°C or 51°C (for polymorphic sites at positions 677 and 1298, respectively), and 45 s at 72°C. PCR products were visualized on 3% agarose gels[10].
The MTHFR 677 (C→T) allele in gastric cancer and CSG tissues, but not the MTHFR 1298 (A→C) allele in gastric cancer samples, was in Hardy-Weinberg equilibrium (P > 0.05).
The transcription levels of tumor-suppressor genes, p16INK4A and p21WAF1; proto-oncogene, c-myc; and mismatch repair (MMR) genes, hMLH1 and hMSH2; were detected using real-time RT-PCR. Total RNA was isolated using a commercial kit (Trizol) according to the manufacturer’s instructions (Invitrogen Gibco BRL, Carlsbad, CA). Reverse transcription reactions using 5 μg of total RNA in a total reaction volume of 20 μL were performed with SuperscriptIIreverse transcriptase (Invitrogen Life Technologies, Inc.). Relative quantitation using the comparative Ct method with data from the ABI PRISM 7700 Sequence Detection System (version 1.6 software) was performed according to the manufacturer’s protocol. The primers and fluorogenic probes for these genes were provided by Shenyou Company, Shanghai. The sequences of the probes and forward and reverse primers are shown in Table 1. Real-time PCR was also performed with primers and a probe for β-actin to normalize each of the extracts for amplifiable human DNA. The results were expressed as the ratio of copies of target genes to β-actin. Ct values were measured, and the average Ct of triplicate samples was calculated. An alteration of mRNA expression was defined as a 3-fold difference in expression level[11].
| Gene | Primer (forward) (5'-3') | Primer (reverse) (5'-3') | Probe | Genbank number |
| β-actin | CTG GCA CCC AGC ACA ATG | GGA CAG CGA GGC CAG GAT | ATC ATT GCT CCT CCT GAG | BC016045 |
| p16INK4A | CAT AGA TGC CGC GGA AGG T | CAG AGC CTC TCT GGT TCT TTC AA | CCT CAG ACA TCC CCG | NM_058197 |
| p21WAF1 | CTG GAG ACT CTC AGG GTC GAA | GGA TTA GGG CTT CCT CTT GGA | ACG GCG GCA GAC CAG CAT GA | NM_078467 |
| c-myc | ACA CCG CCC ACC ACC AG | CCA CAG AAA CAA CAT CGA TTT CTT | AGC GAC TCT GAG GAG G | V00568 |
| hMLH1 | GGC CAG CTA ATG CTA TCA AAG AG | CTT TAA CAA TCA CTT GAA TAC TTG TGG A | ATT GAG AAC TGT TTA GAT GCA | U07418 |
| hMSH2 | ATC CAA GGA GAA TGA TTG GTA TTT G | CAA AGA GAA TGT CTT CAA ACT GAG AGA | CAT ATA AGG CTT CTC CTG GC | U04045 |
| p16INK4A(M) | TTA TTA GAG GGT GGG GCG GAT CGC | GAC CCC GAA CCG CGA CCG TAA | X94154 | |
| p16INK4A(U) | GGG GGA GAT TTA ATT TGG | CCC TCC TCT TTC TTC CTC | X94154 | |
| p21WAF1(M) | TGT AGT ACG CGA GGT TTC G | TCA ACT AAC GCA ACT CAA CG | NM_007592 | |
| p21WAF1(U) | TTT TTG TAG TAT GTG AGG TTT TGG | AAC ACA ACT CAA CAC AAC CCT A | NM_007592 | |
| c-myc (M) | TAG AAT TGG ATC GGG GTA AA | CGA CCG AAA ATC AAC GCG AAT | AF002859 | |
| c-myc (U) | TAG AAT TGG ATT GGG GTA AA | CCA ACC AAA AAT CAA CAT GAA T | AF002859 | |
| hMLH1 (M) | ACG TAG ACG TTT TAT TAG GGT CGC | CCT CAT CGT AAC TAC CCG CG | AB017806 | |
| hMLH1 (U) | TTT TGA TGT AGA TGT TTT ATT AGG GTT GT | ACC ACC TCA TCA TAA CTA CCC ACA | AB017806 | |
| hMSH2 (M) | TCG TGG TCG GAC GTC GTT C | CAA CGT CTC CTT CGA CTA CAC CG | AB006445 | |
| hMSH2 (U) | GGT TGT TGT GGT TGG ATG TTG TTT | CAA CTA CAA CAT CTC CTT CAA CTA CAC CA | AB006445 |
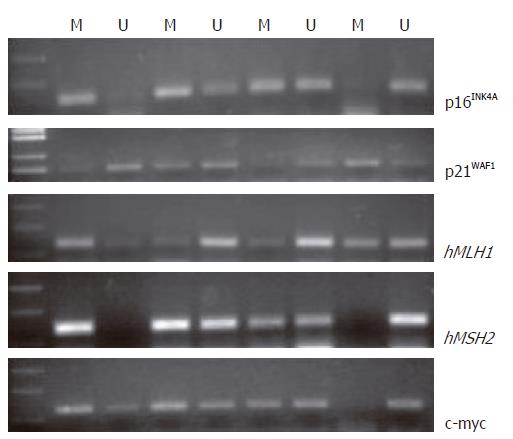
To address whether DNA methylation of tumor-related genes is associated with the folate level in mucosal tissue and MTHFR polymorphisms, methylation-specific PCR (MSP) was performed in CpG-rich regions of p16INK4A and p21WAF1, c-myc, and hMLH1 and hMSH2, in order to detect changes in DNA methylation of the genes due to drug treatments. Bisulfite modification protocols were adopted as described by Xiong and Laird[12]. Genomic DNA treated with bisulfite was amplified with promoter specific primers of each gene (Table 1). The primers were designed without CpG dinucleotides to enable the amplification of both methylated and unmethylated alleles.
The 50 μL PCR reactions consisted of 100 ng of bisulfite-treated DNA, 0.1 mmol/L dNTPs, 2.0 mmol/L MgCl2, and 0.5 μmol/L of each primer. PCR products were directly loaded onto 3% agarose gels and electrophoresed. The gel was stained with ethidium bromide and directly visualized under UV illumination. Wild-type p16INK4A and p21WAF1 primers were used to verify that complete conversion of the DNA occurred in the bisulfite reaction. A positive control for complete methylation was also amplified.
Data were presented as means ± SD. Comparisons between groups were made using Student’s paired t test. The differences between cancerous, non-cancerous and CSG tissues, and their relationships were analyzed by Fisher’s exact test using SAS v.6.12. All statistical tests were two tailed and considered significant at P = 0.05. A case-control study was performed and the allelic frequency of the polymorphism was calculated for both cases and controls. Odds ratios (OR) and 95% CI were calculated to evaluate the association between CSG or cancer and the presence of MTHFR C677T polymorphism. The Mantel Haenszel χ2 procedure was used to assess the linear trend between lesion severity and magnitude of the association with MTHFR C677T genotype.
Folate concentrations in CSG tissue (5.48 ± 2.15 ng/mL) were significantly higher (P < 0.05) than in the other two groups (gastric cancerous tissue, 3.65 ± 1.97 μg/L and corresponding non-cancerous gastric mucosa, 4.01 ± 2.11 μg/L). There were no significant differences in folate concentrations between different sites of the stomach including antrum and corpus. No correlation was found between folate levels and the degree of infiltration, lymph node metastasis, tumor size, or TNM clinical stage of gastric cancer (data not shown).
As shown in Table 2 and Figure 2, decreased expression of c-myc accompanied higher folate levels. However, there was no definite association between folate levels and different expression levels of tumor suppressor genes including p16INK4A, p21WAF1, hMLH1 and hMSH2 in mucosal tissue (data not shown). Furthermore, hypomethylation of c-myc was found in cancerous tissues, which showed up-regulated expression of c-myc, and folate levels in tissues with a hypomethylated c-myc gene showed a downward trend compared with folates in unmethylated samples in advanced gastric cancer (tubular-, mucinous-, and tubular-papillary adenocarcinoma).
| Methylationstatus | Transcription | Casenumber(n) | P1 | Average folateconcentration(μg/L) | P2 |
| Hypomethylated | Up-regulated | 6 | 2.25 ± 1.23 | ||
| Unchanged | 0 | 0.02 | 0.06 | ||
| Methylated | Up-regulated | 13 | 3.91 ± 2.01 | ||
| Unchanged | 19 |
As indicated in Table 3, the 677CC, CT, CC, CT and CC genotypes of MTHFR were most frequently detected with aberrant methylation of p16INK4A, p21WAF1, hMLH1, hMSH2 and c-myc, respectively. The 1298AA genotype was found associated with aberrant methylation of all tumor-related genes studied.
| Genes | MTHFR | Upregulation n (%) | Normal (n) | Downregulation n (%) | mRNA ratio of cancerous to noncancerous tissue (mean ± SD) | P |
| p16INK4A | CC | 1 | 3 | 10 (71.43) | 0.63 ± 1.05 | 0.84 |
| CT | 1 | 8 | 10 (52.63) | 0.7 ± 0.88 | 0.69 | |
| TT | 0 | 2 | 3 (60) | 0.85 ± 0.96 | - | |
| AA | 1 | 9 | 16 (61.54) | 0.57 ± 0.96 | 0.51 | |
| AC | 1 | 4 | 7 (58.33) | 0.81 ± 1.15 | - | |
| p21WAF1 | CC | 1 | 7 | 6 (42.86) | 1.06 ± 1.06 | - |
| CT | 1 | 5 | 13 (68.42) | 0.47 ± 0.69 | 0.062 | |
| TT | 0 | 2 | 3 (60) | 0.55 ± 0.55 | 0.32 | |
| AA | 2 | 10 | 14 (53.85) | 0.75 ± 0.85 | 0.57 | |
| AC | 0 | 4 | 8 (66.67) | 0.58 ± 0.85 | - | |
| c-myc | CC | 6 (42.86) | 7 | 1 | 2.26 ± 2.17 | 0.048 |
| CT | 11 (57.89) | 6 | 2 | 4.32 ± 3.25 | - | |
| TT | 2 (40) | 2 | 1 | 2.15 ± 1.8 | 0.17 | |
| AA | 15 (57.69) | 8 | 3 | 3.84 ± 3.17 | 0.076 | |
| AC | 4 (33.33) | 7 | 1 | 2.06 ± 1.64 | - | |
| hMLH1 | CC | 0 | 3 | 11 (78.57) | 0.46 ± 0.67 | 0.053 |
| CT | 1 | 8 | 10 (52.63) | 0.57 ± 0.82 | 0.13 | |
| TT | 0 | 3 | 1 (25) | 1.3 ± 1.28 | - | |
| AA | 2 | 10 | 14 (53.85) | 0.72 ± 0.96 | 0.28 | |
| AC | 0 | 4 | 8 (66.67) | 0.39 ± 0.55 | - | |
| hMSH2 | CC | 0 | 5 | 9 (64.29) | 0.31 ± 0.32 | - |
| CT | 1 | 10 | 8 (42.11) | 0.88 ± 0.97 | 0.04 | |
| TT | 0 | 3 | 2 (40) | 0.94 ± 1.12 | 0.064 | |
| AA | 1 | 13 | 12 (46.15) | 0.8 ± 0.98 | 0.19 | |
| AC | 0 | 5 | 7 (58.33) | 0.4 ± 0.42 | - |
Compared to the 677CC genotype, expression of hMSH2 was significantly down-regulated in cases with the 677CT genotype, and showed a large but non-significant decrease in the presence of 677TT. Cases with the 677CT genotype showed a lower level of p21WAF1 expression than did cases with the wild-type 677CC genotype, while hMLH1 coupled with the 677CC genotype displayed a trend of decreased expression compared with the 677TT genotype. No differences were observed in p16INK4 mRNA levels between 677CC, 677CT or 677TT. c-myc transcription in cases with the 677CT genotype was significantly higher than when the 677CC genotype was present.
Compared with the 1298AC genotype, c-myc transcription showed an increase in cases with the 1298AA genotype. There was no difference in the expression of p16INK4A, p21WAF1, hMLH1 or hMSH2 between the three MTHFR genotypes.
There were no differences in either the genotype distribution or allele frequency for alleles 677T between gastric cancerous tissue and CSG (P > 0.05), although the 677CT genotype showed a non-significant higher risk for advanced gastric cancer as compared with the 677CC genotype (Table 4). In addition, due to the limitation of sample size, we failed to find any significant association between MTHFR polymorphisms, 677 (C→T) and 1298 (A→C), and the degree of infiltration, lymph node metastasis, tumor size, or clinical stage of gastric cancer (Table 5).
| Polymorphicsite | MTHFRgenotype | Canceroustissue n (%) | CSGn (%) | OR (95% CI) | P |
| C677T | CC | 14 (36.84) | 15 (44.12) | 1 (reference) | - |
| CT | 19 (50.00) | 11 (32.35) | 1.85 (0.65-5.24) | 0.24 | |
| TT | 5 (13.16) | 8 (23.53) | 0.67 (0.17-2.57) | 0.55 | |
| A1298C | |||||
| AA | 26 (68.42) | 22 (64.71) | 1 (reference) | - | |
| AC | 12 (31.58) | 11 (32.35) | 0.92 (0.34-2.52) | 0.87 | |
| CC | 0 | 1 (2.94) | - | - |
| Biological character | 677CC | 677CT | 677TT | 1298AA | 1298AC | |
| Degree of infiltration | Muscularis mucosae | 2 | 2 | 1 | 3 | 2 |
| Muscular layer | 1 | 3 | 2 | 5 | 1 | |
| Serosa | 7 | 9 | 1 | 12 | 5 | |
| Out-serosa | 4 | 5 | 1 | 6 | 4 | |
| Lymph node metastasis | Yes | 7 | 11 | 2 | 14 | 6 |
| No | 7 | 8 | 3 | 12 | 6 | |
| Tumor size | < 5 cm | 9 | 7 | 2 | 11 | 7 |
| ≥ 5 cm | 5 | 12 | 3 | 15 | 5 | |
| TNM classification | I | 2 | 2 | 1 | 3 | 2 |
| II | 7 | 12 | 2 | 16 | 5 | |
| III | 5 | 5 | 2 | 7 | 5 |
The genotypes of 677 and 1298 sites were not associated with folate concentrations in gastric cancerous tissue and CSG (data not shown).
Gastric cancer is a common malignant tumor worldwide, with a much higher incidence in Asian than in Western countries. Multiple genetic and epigenetic alterations are involved in gastric carcinogenesis.
Folate is an important constituent of fruits and vegetables and may confer protection against cancer. An important biological function of folate is to provide methyl groups required for intracellular methylation reactions and de novo deoxynucleoside triphosphate synthesis; therefore, folate deficiency is thought to be carcinogenic through disruption of DNA methylation and synthesis and impaired DNA repair[13]. However, folate requires metabolic transformations catalyzed by several enzymes including MTHFR, which irreversibly converts 5, 10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. The MTHFR gene is highly polymorphic, of which the two most common variants are located at nucleotides 677 (C→T) and 1298 (A→T). Low MTHFR activity may prevent the shunting of methyl groups from de novo dTMP synthesis, a rate-limiting step for DNA synthesis, to methylation pathways.
It has been suggested that the cancer risk associated with MTHFR polymorphisms may be modulated by folate intake[14]. When folate intake is sufficient, individuals carrying the variant MTHFR genotypes may have a decreased risk because under these conditions, while adequate provision of methyl donors is still ensured, enhanced genomic integrity would be achieved via conservation of folate within a cyclic intracellular pathway by shunting methyl groups toward nucleotide synthesis due to diminished MTHFR activity. However, when folate intake is low, both DNA methylation and DNA synthesis and repair might be impaired in carriers of variant MTHFR genotypes, which, in turn, results in increased risk of carcinogenesis. This hypothesis of a gene-nutrient interaction may explain the conflicting reports showing either reduced[10,15,16] or elevated[17,18] risk of cancers.
Many previous studies have investigated the relationship between folate status and MTHFR gene poly-morphisms in carcinogenesis. However, because they were mainly focused on blood plasma and blood cells, little is known about folate status in mucosal tissues. Gastric cancer originates from epithelial cells; therefore, a study performed in mucosal tissues is more accurate and effective than those performed using blood. In the present study, we examined folate concentrations and MTHFR gene polymorphisms in gastric mucosal tissues, and found that folate levels were significantly lower in cancer cases (including cancerous and corresponding non-cancerous tissues) than that in the CSG controls (no chronic atrophic gastritis, intestinal metaplasia or dysplasia), suggesting that folate deficiency may increase the risk of cancer[4]. However, we failed to find the association between folate levels and the degree of infiltration, lymph node metastasis, tumor size, or clinical stage of gastric cancer. Possibly folate levels in mucosal tissue influence the initiation but not the progression of gastric cancer.
Transcriptional silencing of tumor suppressor genes by DNA hypermethylation and over-expression of proto-oncogenes by DNA hypomethylation play crucial roles in the progression of gastric cancer. Many genes involved in the regulation of cell cycle, tissue invasion, DNA repair and apoptosis have been shown to be inactivated by this type of epigenetic mechanism. The loss of p16INK4A expression, and hypermethylation in the promoter region is the mechanism of loss of p21WAF1, and hypermethylation of the hMLH1 gene promoter has been associated with a transcriptional blockade. The fact that the blockade is reversible with demethylation suggests that an epigenetic mechanism underlies hMLH1 gene inactivation and MMR genes deficiency[19]. However, the role of DNA methylation in the loss of hMSH2 expression has been controversial[20,21]. Aberrant methylation of c-myc can induce over-expression of the gene, and participate in the development of tumors. Hypomethylation of c-myc has been detected in gastric carcinogenesis[22], and it has been reported that folate[23] and MTHFR gene polymorphisms[24] are associated with aberrant methylation of some tumor-related genes.
Miao et al[25] revealed that the 677TT genotype is associated with an increased risk of gastric cardia cancer. However, our data showed that MTHFR polymorphisms may not be an independent factor affecting initiation and progression of gastric cancers, including antrum and corpus cancers. In addition, due to the limit of sample size, we could not find a significant association between the MTHFR polymorphisms [677 (C→T) and 1298 (A→C)] and the degree of infiltration, lymph node metastasis, tumor size, or clinical stage of gastric cancer. Folate levels but not MTHFR polymorphisms affect the methylation and expression of proto-oncogene or tumor suppressor genes related to human gastric carcinogenesis, although it is unclear how low folate levels lead to c-myc hypomethylation and its down-regulated expression.
In summary, a folate level reduction was observed in gastric cancer tissues. This change, but not methylenetetrahydrofolate reductase polymorphisms, is associated with upregulation of c-myc expression and hypomethylation of its promoter region.
Multiple genetic and epigenetic alterations are involved in gastric carcinogenesis. Folate deficiency is thought to be carcinogenic through disruption of DNA methylation and synthesis and impaired DNA repair. However, folate requires metabolic transformations catalyzed by several enzymes including MTHFR, which is highly polymorphic.
The present study discussed the relationship between folate concentrations in mucosal tissues, MTHFR gene polymorphisms and expression of tumor-related genes, as well as DNA methylation in human gastric carcinogenesis.
Gastric cancer originates from epithelial cells, therefore, a study performed in mucosal tissues is more accurate and effective than those performed in blood. The extent to which folate tissue levels and MTHFR polymorphisms interact to affect DNA methylation in gastric carcinogenesis is uncertain.
The present study investigated the etiology of gastric carcinogenesis, which may provide the evidence for prevention and treatment of gastric cancer.
Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in folate metabolism that regulates the intracellular folate pool. The MTHFR gene is highly polymorphic, of which the two most common variants are located at nucleotides 677 (C→T) and 1298 (A→T).
The authors evaluated whether folate level in mucosal tissue and some common MTHFR variants are associated with the risk of gastric cancer through an effect on DNA methylation. The figures are clear. However, the title and conclusion need to be more concise and exact.
S- Editor Wang GP L- Editor Zhu LH E- Editor Bai SH
| 1. | Fang JY, Xiao SD. Alteration of DNA methylation in gastrointestinal carcinogenesis. J Gastroenterol Hepatol. 2001;16:960-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Fang JY, Xiao SD, Zhu SS, Yuan JM, Qiu DK, Jiang SJ. Relationship of plasma folic acid and status of DNA methylation in human gastric cancer. J Gastroenterol. 1997;32:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Fang JY, Zhu SS, Xiao SD, Jiang SJ, Shi Y, Chen XY, Zhou XM, Qian LF. Studies on the hypomethylation of c-myc, c-Ha-ras oncogenes and histopathological changes in human gastric carcinoma. J Gastroenterol Hepatol. 1996;11:1079-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:1055-1062. [PubMed] [Cited in This Article: ] |
| 5. | Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U.S. Cancer. 1997;80:1021-1028. [PubMed] [Cited in This Article: ] |
| 6. | Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001;61:3272-3275. [PubMed] [Cited in This Article: ] |
| 7. | Kim JK, Kim S, Han JH, Kim HJ, Chong SY, Hong SP, Hwang SG, Ahn JY, Cha KY, Oh D. Polymorphisms of 5,10-methylenetetrahydrofolate reductase and risk of stomach cancer in a Korean population. Anticancer Res. 2005;25:2249-2252. [PubMed] [Cited in This Article: ] |
| 8. | Graziano F, Kawakami K, Ruzzo A, Watanabe G, Santini D, Pizzagalli F, Bisonni R, Mari D, Floriani I, Catalano V. Methylenetetrahydrofolate reductase 677C/T gene polymorphism, gastric cancer susceptibility and genomic DNA hypomethylation in an at-risk Italian population. Int J Cancer. 2006;118:628-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Shen H, Newmann AS, Hu Z, Zhang Z, Xu Y, Wang L, Hu X, Guo J, Wang X, Wei Q. Methylenetetrahydrofolate reductase polymorphisms/haplotypes and risk of gastric cancer: a case-control analysis in China. Oncol Rep. 2005;13:355-360. [PubMed] [Cited in This Article: ] |
| 10. | Krajinovic M, Lamothe S, Labuda D, Lemieux-Blanchard E, Theoret Y, Moghrabi A, Sinnett D. Role of MTHFR genetic polymorphisms in the susceptibility to childhood acute lymphoblastic leukemia. Blood. 2004;103:252-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Scanlan MJ, Welt S, Gordon CM, Chen YT, Gure AO, Stockert E, Jungbluth AA, Ritter G, Jäger D, Jäger E. Cancer-related serological recognition of human colon cancer: identification of potential diagnostic and immunotherapeutic targets. Cancer Res. 2002;62:4041-4047. [PubMed] [Cited in This Article: ] |
| 12. | Xiong Z, Laird PW. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25:2532-2534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 913] [Cited by in F6Publishing: 874] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 13. | Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130:129-132. [PubMed] [Cited in This Article: ] |
| 14. | Fang JY, Xiao SD. Folic acid, polymorphism of methyl-group metabolism genes, and DNA methylation in relation to GI carcinogenesis. J Gastroenterol. 2003;38:821-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Zoodsma M, Nolte IM, Schipper M, Oosterom E, van der Steege G, de Vries EG, te Meerman GJ, van der Zee AG. Methylenetetrahydrofolate reductase (MTHFR) and susceptibility for (pre)neoplastic cervical disease. Hum Genet. 2005;116:247-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Ulvik A, Vollset SE, Hansen S, Gislefoss R, Jellum E, Ueland PM. Colorectal cancer and the methylenetetrahydrofolate reductase 677C -> T and methionine synthase 2756A -> G polymorphisms: a study of 2,168 case-control pairs from the JANUS cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:2175-2180. [PubMed] [Cited in This Article: ] |
| 17. | Stolzenberg-Solomon RZ, Qiao YL, Abnet CC, Ratnasinghe DL, Dawsey SM, Dong ZW, Taylor PR, Mark SD. Esophageal and gastric cardia cancer risk and folate- and vitamin B(12)-related polymorphisms in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2003;12:1222-1226. [PubMed] [Cited in This Article: ] |
| 18. | Piyathilake CJ, Macaluso M, Johanning GL, Whiteside M, Heimburger DC, Giuliano A. Methylenetetrahydrofolate reductase (MTHFR) polymorphism increases the risk of cervical intraepithelial neoplasia. Anticancer Res. 2000;20:1751-1757. [PubMed] [Cited in This Article: ] |
| 19. | Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, Zou TT, Abraham JM, Kong D, Smolinski KN. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999;59:1090-1095. [PubMed] [Cited in This Article: ] |
| 20. | Kim HC, Kim CN, Yu CS, Roh SA, Kim JC. Methylation of the hMLH1 and hMSH2 promoter in early-onset sporadic colorectal carcinomas with microsatellite instability. Int J Colorectal Dis. 2003;18:196-202. [PubMed] [Cited in This Article: ] |
| 21. | Saito T, Oda Y, Kawaguchi K, Takahira T, Yamamoto H, Sakamoto A, Tamiya S, Iwamoto Y, Tsuneyoshi M. Possible association between tumor-suppressor gene mutations and hMSH2/hMLH1 inactivation in alveolar soft part sarcoma. Hum Pathol. 2003;34:841-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Fang J, Zhu S, Xiao S, Shi Y, Jiang S, Zhou X, Qian L. Alterations of level of total genomic DNA methylation and pattern of c-myc, c-Ha-ras oncogene methylation in human gastric carcinogenesis. Chin Med J (Engl). 1996;109:787-791. [PubMed] [Cited in This Article: ] |
| 23. | van Engeland M, Weijenberg MP, Roemen GM, Brink M, de Bruïne AP, Goldbohm RA, van den Brandt PA, Baylin SB, de Goeij AF, Herman JG. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: the Netherlands cohort study on diet and cancer. Cancer Res. 2003;63:3133-3137. [PubMed] [Cited in This Article: ] |
| 24. | Oyama K, Kawakami K, Maeda K, Ishiguro K, Watanabe G. The association between methylenetetrahydrofolate reductase polymorphism and promoter methylation in proximal colon cancer. Anticancer Res. 2004;24:649-654. [PubMed] [Cited in This Article: ] |
| 25. | Miao X, Xing D, Tan W, Qi J, Lu W, Lin D. Susceptibility to gastric cardia adenocarcinoma and genetic polymorphisms in methylenetetrahydrofolate reductase in an at-risk Chinese population. Cancer Epidemiol Biomarkers Prev. 2002;11:1454-1458. [PubMed] [Cited in This Article: ] |