Published online Nov 14, 2006. doi: 10.3748/wjg.v12.i42.6812
Revised: August 16, 2006
Accepted: August 27, 2006
Published online: November 14, 2006
AIM: To evaluate the protective effects of preconditioning by α-lipoic acid (LA) in patients undergoing hepatic resection under inflow occlusion of the liver.
METHODS: Twenty-four patients undergoing liver resection for various reasons either received 600 mg LA or NaCl 15 min before transection performed under inflow occlusion of the liver. Blood samples and liver wedge biopsy samples were obtained after opening of the abdomen immediately after inflow occlusion of the liver, and 30 min after the end of inflow occlusion of the liver.
RESULTS: Serum levels of aspartate transferase and alanine transferase were reduced at all time points in patients who received LA in comparison to those who received NaCL. This was accompanied by reduced histomorphological features of oncosis. We observed TUNEL-positive hepatocytes in the livers of the untreated patients, especially after 30 min of ischemia. LA attenuated this increase of TUNEL-positive hepatocytes. Under preconditioning with LA, ATP content was significantly enhanced after 30 min of ischemia and after 30 min of reperfusion.
CONCLUSION: This is the first report on the potential for LA reducing ischemia/reperfusion injury (IRI) of the liver in humans who were undergoing liver surgery. Beside its simple and rapid application, side effects did not occur. LA might therefore represent a new strategy against hepatic IRI in humans.
- Citation: Dünschede F, Erbes K, Kircher A, Westermann S, Seifert J, Schad A, Oliver K, Kiemer AK, Theodor J. Reduction of ischemia reperfusion injury after liver resection and hepatic inflow occlusion by α-lipoic acid in humans. World J Gastroenterol 2006; 12(42): 6812-6817
- URL: https://www.wjgnet.com/1007-9327/full/v12/i42/6812.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i42.6812
Excessive blood loss during surgery and the need for transfusion have been shown to hinder the post-operative course of patients[1]. In order to minimize blood loss, clamping of the portal triad (inflow occlusion), also called pringle maneuvre, is used during liver surgery[2]. However, the risk of ischemia/reperfusion injury (IRI) of the liver is increased under these conditions[3].
Ischemia reperfusion injury of the liver is still a major cause of morbidity and mortality in patients undergoing liver surgery and transplantation[4]. A period of ischemia followed by a brief period of ischemia (ischemic preconditioning, IP) prior to sustained ischemia is a method to reduce IRI of the liver in animal models as well as in humans[5]. However, the protective effects are lost in older patients and in patients undergoing major tissue loss (> 50% of the liver)[6].
In animal models there are numerous pharmacological agents protecting the liver from IRI[7]. However, there is a lack of clinically proven strategies to pharmacologically reduce IRI of the liver in humans. We have previously described the potential of α-lipoic acid (LA) to protect from IRI of the isolated perfused rat liver, which could be confirmed in vivo for livers undergoing 90 min of warm ischemia[8].
α-Lipoic acid is a naturally occurring compound present in the majority of pro- and eukaryotic cells. For many years LA has been used as a pharmacological agent against diabetic polyneuropathy and is known to be without serious side effects[9]. Therefore, oral and intravenous application as well as the bioavailability of LA is well established[10]. The aim of the present study was to evaluate the influence of LA in ischemia/reperfusion injury of the liver in patients who are undergoing liver resection.
Informed consent was obtained from each patient before surgery. Patients scheduled to undergo liver resection were included. Characteristics of patients are shown in Table 1. Baseline wedge biopsy samples were taken from the part of the liver to be resected. Clamping of the portal triad was performed with the torniquet technique. Samples of liver tissue (wedge biopsy) obtained during surgery were immediately transferred into liquid nitrogen or stored in 4% paraformaldehyde. Separate clamping of aberrant left hepatic arteries was carefully performed when present. Transection of the liver was immediately performed after complete hepatic inflow occlusion; if necessary vessels and bile duct were ligated with 2-0 silk suture. After exactly 30 min, the portal clamping was released and hemostasis was obtained. Each surgery was performed with a low central venous pressure to minimize blood loss and was carried out by using the same anesthetic combinations. Surgery was performed by one surgeon.
| characteristics | Vehicle | LA |
| Age (yr) | 61 (34-79) | 63 (46-79) |
| Sex (M/F) | 5/7 | 6/6 |
| Resected liver tissue (g) | 434 (40-1100) | 588 (112-1365) |
| Blood loss (mL) | 1175 (400-2000) | 825 (200-2200) |
| RC-transfusion intraoperative (mL) | 300 (0-1000) | 265 (0-1000) |
| Intensive care unit (d) | 1 (0-7) | 2 (0-4) |
| Hospital stay (d) | 13 (6-21) | 14 (11-25) |
| Post-OP complications | 1/12 | 1/12 |
| Mortality | 1/12 | 0/12 |
| Indications, malignant | 11/12 | 11/12 |
Our protocol is based on the data we obtained in rodents, where an amount of 500 μmol (120 μg) LA strongly protected the liver against IRI after a prolonged period of ischemia[8]. Previous studies have shown that ischemic injury in humans becomes detectable after 30 min of ischemia[11]. Twenty-four patients were subjected either to preconditioning with 600 mg LA (in 50 mL NaCl) intravenously 15 min before inflow occlusion of the liver or to receive 50 mL NaCl intravenously 15 min before inflow occlusion of the liver. We performed ischemia 15 min after ending the LA injection because its bioavailability is sufficient 15 min after intravenous application[10].
In order to minimize bias, LA preconditioning was performed in an alternate fashion. Patients with cirrhosis, elevated liver enzymes as well as high operation risk were excluded. Blood samples and wedge biopsies were taken after opening the abdomen (LAP), after 30 min of ischemia (pP0´), and after 30 min of reperfusion (pP30`). Blood samples were taken every day until discharge. All blood samples were analyzed using a serum analyzer (Olympus AU Connector 640, Hamburg, Germany). Wedge biopsy samples were immediately stored in liquid nitrogen or fixed for histological examination. Liver tissue was weighed prior to homogenization and diluted 1:5 with 0.01 mmol/L PBS (phosphate buffered saline). Homogenization was then carried out with a Potter liver homogenizer at 800 revolutions/mine. The mixture was centrifuged at 1000 × g for 10 min, the supernatant was transferred into an Eppendorf cup and centrifuged at 10 000 × g for 20 min. The clear supernatant was pipetted into a fresh Eppendorf cup, and stored until further measurement.
Measurement of adenosine triphosphate (ATP) in liver tissue was carried out using a standardized method[12]. The liver slices were weighed and diluted in 4 % HClO4 (1:10) prior to homogenization with a Potter liver homogenizer at 850 revolutions/min. The mixture was centrifuged at 10 000 × g for 5 min at 4°C. The supernatant was pipetted into an Eppendorf cup and neutralized with 150 μL 2 mol/L Tris, 150 μL KOH, and phenol-red. 5 mol/L KOH was then added until a blew-lilac complex was observed. The mixture was again centrifuged at 10 000 U/min for 5 min at 4°C. The supernatant was measured immediately as described by Trautschold et al. with a hexokinase/glucose-6-phosphat dehydrogenase reaction by UV photometry[13].
Formalin-fixed tissue samples were embedded in paraffin, and 5 μm sections were cut. Replicate sections were stained with hematoxylin and eosin (H&E) for the evaluation of oncosis. Morphological criteria of oncosis, such as increased swelling, vacuolization and blebbing were assessed. The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was employed for the evaluation of apoptotic related cell injury. For TUNEL staining, livers were fixed with freshly prepared 4% paraformaldehyde in neutral buffered saline (PBS). Frozen sections (5 μm) of the fixed tissue were prepared and stained with the TUNEL method using a commercially available kit (Chemicon International, ApopTag peroxidase apoptosis detection kit, Hampshire, UK). The number of TUNEL-positve hepatocytes was counted in 10 high power fields (400 fold magnification). All histological evaluations were done in a blinded fashion.
All data are expressed as means ± standard deviation. Statistical differences between experimental groups were calculated by Graph Pad Prism version 4.00 for Windows, GraphPad Software, San Diego California USA 1994-1999, and P < 0.05 was considered significant. One-way analysis of variance (ANOVA) with subsequent post hoc Tukey-test was used.
There was no statistical difference between the two groups indicating that they were homogeneous and comparable (Table 1, data were expressed with median values with ranges [minimum-maximum]). One patient in the untreated group died because he suffered mesenteric ischemia at day 10 after liver resection. During revision of the abdomen we saw great areas of necrotic small intestine whereas the portal vein was not occluded. Operative morbidity was low in both groups. One patient in each group suffered bile leakage; both were cured by local drainage.
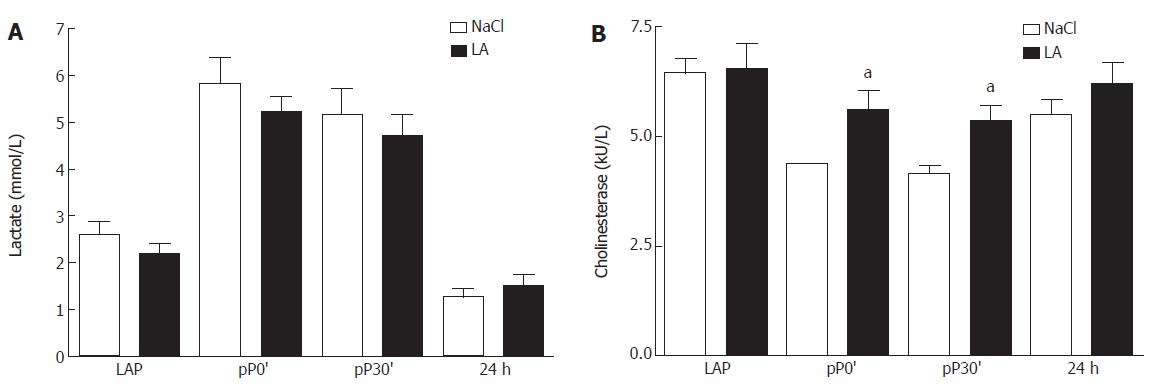
To evaluate inflow occlusion in both groups we determined lactate levels in serum. We observed no difference of lactate between both groups directly after opening of the abdomen (LAP), after 30 min of ischemia, after 30 min of reperfusion, and 24 h after inflow occlusion (Figure 1A).
Cholinesterase (CHE) in serum was decreased in the untreated group at pP0´and pP30` compared to LAP while levels of CHE were significantly increased at both time points in LA-treated patients compared to controls (Figure 1B).
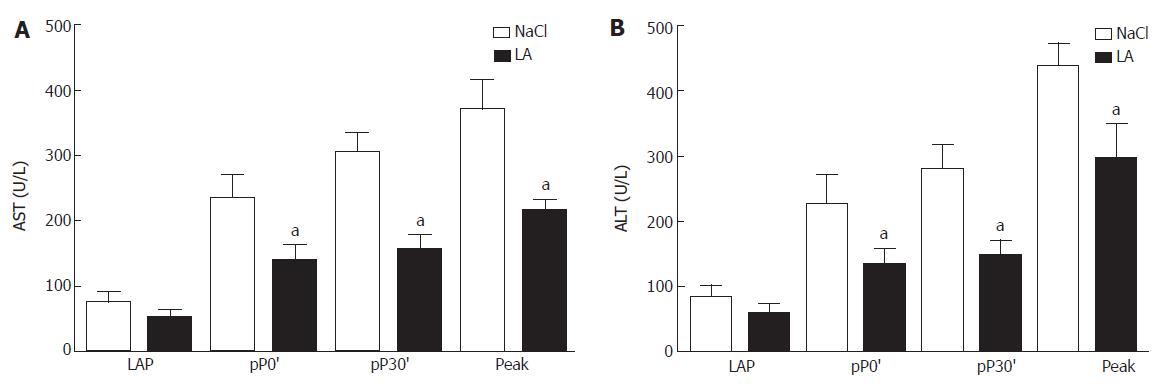
After opening the abdomen AST levels in serum were low in both groups. After 30 min of ischemia, at 30 min of reperfusion, as well as at days 1-3 after the operation (peak level) the value increased in the untreated patients. In the LA-pretreated group we measured significantly lower levels of AST at each time point (Figure 2A). AST and ALT levels in both groups reached baseline values until discharge (Figure 2B). Total bilirubin (tBIL) and glucose (GC) did not differ from baseline levels neither in controls nor in LA-treated patients (data not shown).
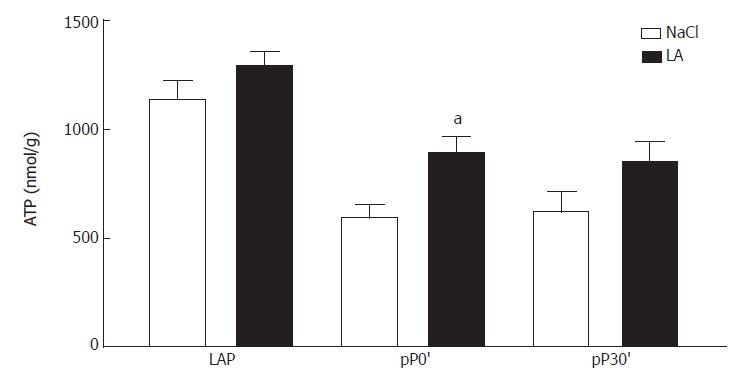
After pretreatment by LA we observed significantly increased ATP content in post-ischemic liver tissue compared to the untreated group (Figure 3). During reperfusion ATP content was also increased in the LA group but did not reach statistical significance.
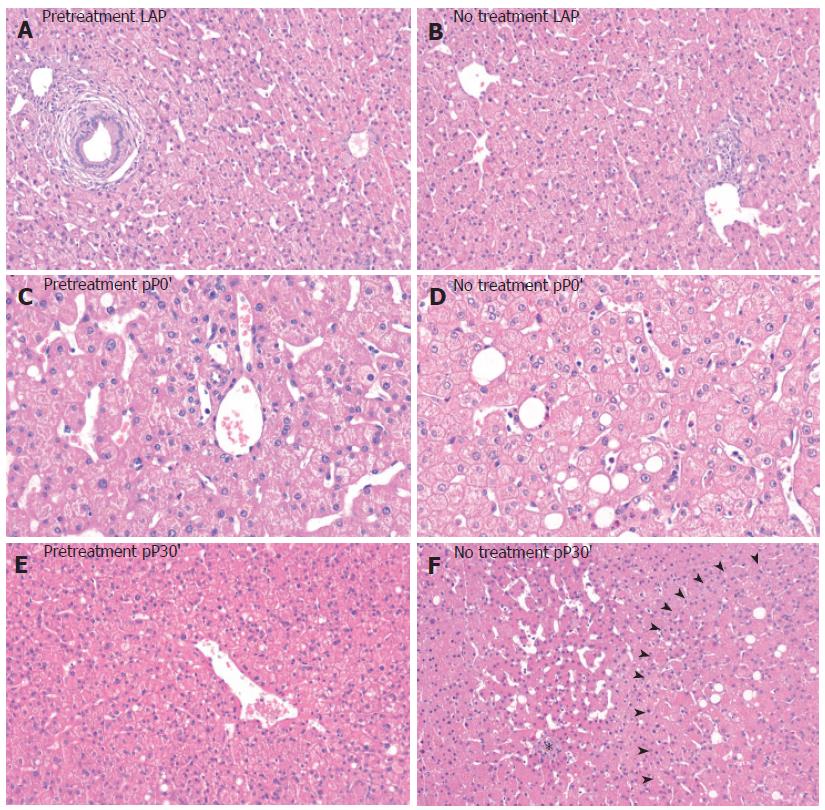
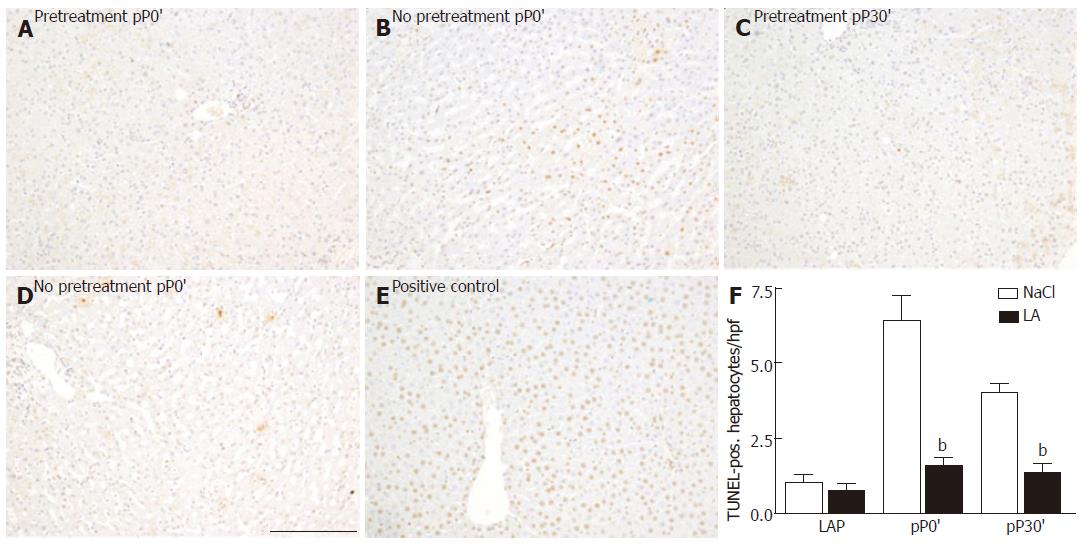
Our histological findings strongly support our results regarding the enzyme release of AST and ALT. H&E-stained liver tissue of untreated patients clearly showed histomorphological features of oncocis (Figure 4). In contrast, in livers of the LA-pretreated patients features of oncocis were rare. We observed TUNEL-positive hepatocytes after 30 min of ischemia (pP0´) in the untreated group as well as at pP30`. In the LA-pretreated group TUNEL-positive hepatocytes were significantly reduced (Figure 5).
Our data strongly support the hypothesis that LA reduces hepatic IRI in humans after inflow occlusion and liver resection. The protection is facilitated by preservation of ATP levels in liver tissue and characterized by reduced apoptotic hepatocyte injury upon LA preconditioning.
LA is established in the therapy of diabetic polyneuropathy[14]. It is a safe substance without serious side effects. Although LA is known to increase blood sugar utilization[12] we did not observe any changes in blood sugar during our study after LA administration (data not shown). LA predominantly undergoes β-oxidation and methylation in the liver which is why it is subjected to a high hepatic extraction[15].
Until now there is a lack of clinical trials that describe pharmacological preconditioning of the liver to protect against IRI. Moreover, established strategies, such as IP, lose their potential in patients with age over 60 years or in cases of high volume liver resection[6]. Our current pilot study points to LA as a potential strategy to protect against hepatic IRI of the liver. We observed histomorphological increased features of oncosis in the non-treated group accompanied by significantly increased levels of enzyme release into plasma. Interestingly, hepatocyte injury clearly occurs in the course of ischemia as it has previously been shown in experimental animal studies[16]. This finding supports the necessity for interventions against IRI to begin before the onset of ischemia. Strategies that concentrate on reperfusion of the liver, such as glutathione, may not be sufficient because the initial damage has already started[17].
The clamping period was set exactly at 30 min. This period is the shortest ischemic time associated with elevated transaminase levels after surgery[11]. The lack of significant effects of pretreatment by LA on patient outcome and other parameters of hepatic injury may be related to this short period of ischemia. On the other hand we could demonstrate that LA improves cholinesterase activity in serum. Former studies have shown increased activity of cholinesterase and liver function in patients with compensated liver cirrhosis after LA treatment[18]. We therefore suggest that LA pretreatment also protects against IRI in patients with liver disease, such as cirrhosis or steatosis.
The detailed mechanism of action of LA remains speculative. We observed increased levels of ATP in liver tissue in the LA group accompanied by improved liver histology. One possible explanation for this result may be the known potential of LA to reform vicinal thiol groups in the ATP synthase. Dithiols have been shown to form disulfide bridges during ischemia/reperfusion. ATP synthesis occurs only if the dithiols between the F1 and the F0- part of the enzyme remain functional[19]. Since ATP depletion is known to induce cellular swelling and other features of oncotic and necrotic cell injury[20], LA might decrease hepatic injury by increased levels of ATP.
We observed that LA decreases apoptotic related cell death. The influence of apoptotic cell death on IRI of the liver in humans has been described[4]. The underlying mechanism of LA to reduce apoptotic related cell death was not within the scope of this study. However, results of our studies in rats showed that LA influences pro- and anti-apoptotic proteins towards the anti-apoptotic Bcl-2 (unpublished data). LA might therefore improve expression of anti-apoptotic proteins, such as Bcl-2 but there is a need for respective investigations in the clinical setting.
In summary, we report for the first time the protection of hepatic IRI after liver surgery and hepatic inflow occlusion by a single infusion of LA 15 min prior to ischemia. This strategy might represent a cost effective, safe and efficient strategy to attenuate ischemia reperfusion injury of the liver in the clinical setting.
The excellent technical assistance of Ms. Müller and Ms. Molter of the Institute of Pathology is gratefully appreciated. We thank the Braun Stiftung for support of the project. Presented in abstract form at the 41st Congress of the European Society for Surgical Research, 2006, Rostock (Germany) and the 123rd Congress of the German Society for Surgery, 2006, Berlin (Germany).
S- Editor Pan BR L- Editor Luzte M E- Editor Bi L
| 1. | Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740-748; discussion 748-749;. [PubMed] [Cited in This Article: ] |
| 2. | Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 855] [Cited by in F6Publishing: 819] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 3. | Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 420] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 339] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Rüdiger HA, Kang KJ, Sindram D, Riehle HM, Clavien PA. Comparison of ischemic preconditioning and intermittent and continuous inflow occlusion in the murine liver. Ann Surg. 2002;235:400-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843-850; discussion 851-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 391] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 7. | Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 417] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Müller C, Dünschede F, Koch E, Vollmar AM, Kiemer AK. Alpha-lipoic acid preconditioning reduces ischemia-reperfusion injury of the rat liver via the PI3-kinase/Akt pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G769-G778. [PubMed] [Cited in This Article: ] |
| 9. | Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schütte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia. 1995;38:1425-1433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 314] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Teichert J, Kern J, Tritschler HJ, Ulrich H, Preiss R. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:625-628. [PubMed] [Cited in This Article: ] |
| 11. | Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, Marty J, Farges O. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 362] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Ramrath S, Tritschler HJ, Eckel J. Stimulation of cardiac glucose transport by thioctic acid and insulin. Horm Metab Res. 1999;31:632-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Trautschold I, Lamprecht W, Schweitzer G. ATP: UV-method with hexokinase and glucose-6-phosphate dehydrogenase. Methods of enzymatic analysis. New York: Academic Press 1988; 346-357. [Cited in This Article: ] |
| 14. | Reljanovic M, Reichel G, Rett K, Lobisch M, Schuette K, Möller W, Tritschler HJ, Mehnert H. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy. Free Radic Res. 1999;31:171-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Hermann R, Niebch G. Human pharmacokinetics of -lipoic acid. Lipoic Acid in Health and Disease. New York: Marcel Dekker 1997; 337-359. [Cited in This Article: ] |
| 16. | Soeda J, Miyagawa S, Sano K, Masumoto J, Taniguchi S, Kawasaki S. Cytochrome c release into cytosol with subsequent caspase activation during warm ischemia in rat liver. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1115-G1123. [PubMed] [Cited in This Article: ] |
| 17. | Bilzer M, Baron A, Schauer R, Steib C, Ebensberger S, Gerbes AL. Glutathione treatment protects the rat liver against injury after warm ischemia and Kupffer cell activation. Digestion. 2002;66:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | BREUER H, SCHOENFELDER M, SCHREIBER HW, BURGMANN W. [Effect of thioctic acid on the activity of different serum enzymes in chronic liver diseases]. Acta Hepatosplenol. 1962;9:104-111. [PubMed] [Cited in This Article: ] |
| 19. | Dünschede F, Zwicker K, Ackermann H, Zimmer G. ADP- and oligomycin-sensitive redox behavior of F0 b thiol in ATPsynthase depends on neighbored primary structure: investigations using 14-C-labeled alpha lipoic acid. Biofactors. 2003;19:19-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Nieminen AL, Gores GJ, Wray BE, Tanaka Y, Herman B, Lemasters JJ. Calcium dependence of bleb formation and cell death in hepatocytes. Cell Calcium. 1988;9:237-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 2.8] [Reference Citation Analysis (0)] |