Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6715
Revised: September 1, 2006
Accepted: September 20, 2006
Published online: November 7, 2006
AIM: To observe the efficiency and safety of thymosin-α1 treatment in patients with hepatitis B e antigen (HBeAg) and HBV DNA positive chronic hepatitis.
METHODS: Sixty-two patients were randomly divided into groups A and B. The patients in group A received subcutaneous injection of 1.6 mg thymosin-α1, twice a week (T-α1 group) for six months, and the patients in group B received 5 MU interferon alpha (IFN-α) each day for fifteen days, then three times weekly (IFN-α group) for six months. The results between two groups treated with and the group untreated with IFN-α which was followed up for 12 mo (historical control group consisting of 30 patients) were compared, and three groups were comparable between each other (P > 0.05) at baseline (age, sex, clinical history, biochemical, and serological parameters).
RESULTS: At the end of treatment, complete response, which was defined as alanine aminotransferase (ALT) normalization and HBV DNA and HBeAg loss, occurred in 9 of 29 (31.0%) patients in the T-α1 group and in 15 of 33 (45.5%) patients in the IFN-α group (χ2 = 1.36, P > 0.05). After a follow-up period of six months, a complete response was observed in 14 of 29 (48.3%) patients in the T-α1 group and in 9 of 33 (27.3%) patients in the IFN-α group (χ2 = 2.93, P > 0.05). Compared with the results observed in the historical control (HC) group untreated with IFN-α which was followed up for 12 mo, the rate of complete response was significantly higher in IFN-α group at the end of therapy (1 of 30 vs 15 of 33, χ2 = 14.72, P < 0.001) and in the T-α1 group at the end of follow-up (1 of 30 vs 14 of 29, χ2 = 15.71, P < 0.001). In T-α1 and IFN-α treatment groups, the area under (the plasma concentration time) curve (AUC) of negative HBV DNA and HBeAg was 34%, 17%, 31% and 19% smaller than that in the HC group. By the end of the follow-up period, the proportions of ALT normalization and negative HBV DNA in the T-α1 group were significantly higher than those in the IFN-α and HC groups. The odds of ALT normalization and negative HBV DNA at the end of the follow-up was three-fold higher in the T-α1 group than in the IFN-α group. Unlike IFN-α, T-α1 was well tolerated by all patients, and no side effects appeared in T-α1 group.
CONCLUSION: The results suggest that a 6-mo course of T-α1 therapy is effective and safe in patients with chronic hepatitis B. T-α1 is able to reduce HBV replication in patients with chronic hepatitis B. Furthermore, T-α1 is better tolerated than IFN-α and can gradually induce more sustained ALT normalization and HBV DNA and HBeAg loss. However, a response rate of 48.3% is still less ideal. A more effective therapeutic approach warrants further study.
- Citation: You J, Zhuang L, Cheng HY, Yan SM, Yu L, Huang JH, Tang BZ, Huang ML, Ma YL, Chongsuvivatwong V, Sriplung H, Geater A, Qiao YW, Wu RX. Efficacy of thymosin alpha-1 and interferon alpha in treatment of chronic viral hepatitis B: A randomized controlled study. World J Gastroenterol 2006; 12(41): 6715-6721
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6715.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6715
Chronic hepatitis B virus (HBV) infection is a serious problem worldwide and may result in adverse sequelae, such as cirrhosis and hepatocellular carcinoma (HCC)[1-2], which is becoming more prevalent worldwide, especially in HBV-endemic areas[3-4]. According to the World Health Organization estimation, more than 350 million people are infected with HBV worldwide, of them up to 20% will become chronic HBV carriers, and 50% would develop chronic liver disease with a significant risk of developing liver cirrhosis and HCC[4]. Despite the introduction of universal vaccination against hepatitis B in over 100 countries, persistent HBV infection is still a serious problem worldwide, causing one million deaths each year[4-6]. It may take several decades to reduce its transmission and morbidity by vaccination. Meanwhile, patients with persistent HBV infection require better antiviral therapeutic modalities than the currently available ones[4]. The ultimate goal of therapy for chronic hepatitis B is to prevent its progression to cirrhosis and development of HCC. Recent prospective studies from Taiwan indicate that higher plasma HBV DNA levels in persons infected with HBV are associated with increased risk of developing HCC, reinforcing the importance of controlling viral replication among patients with chronic hepatitis B[7-10].
Over the past 20 years, many antiviral or immunomo-dulatory agents have been used in the treatment of chronic HBV infection[11-20]. Among them, interferon alpha (IFN-α), has been shown to be effective against chronic HBV infection, and induces an apparent initial response in approximately 30%-40% of treated patients[21-23]. However, the response rate is far from satisfactory, particularly in Asian patients. The relapse rate after treatment withdrawal is high[24].
Lamivudine suppresses viral replication, resulting in HBeAg seroconversion in 16%-17% of patients and histologic improvement in 52%-56% patients after one year of therapy[25,26]. However, within four years, the rate of emergence of lamivudine-resistant HBV mutants approaches 70%[27-30], which is usually associated with a rebound in viral load and exacerbation of hepatitis[31-34]. Pegylated-interferon alfa-2a (IFNα- 2a) appears to have a better efficacy than lamivudine HBeAg seroconversion rate was 32%, 27% and 19%, respectively in pegylated- IFNα-2a monotherapy, plus Lamivudine and lamivudine monotherapy; alanine aminotransferase (ALT) normalization was 41%, 39% and 28%, respectively in HBeAg-positive patients 6 mo after 48-wk treatment. However, the rate of adverse events associated with pegylated IFNα-2a is higher than that of lamivudine[35]. Patients with HBeAg-negative chronic hepatitis B treated with pegylated IFNα-2a have a significantly higher rate of response which could sustain for 24 wk after the therapy (monotherapy or in combination with lamivudine) than those treated with lamivudine monotherapy. After 24 wk of follow-up, the percentages of patients with ALT normalization or HBV DNA level below 20 000 copies/mm3 are 59%, 43%, 60% and 44% vs 44% (P = 0.004 and P = 0.003) and 29% (P = 0.007 and P = 0.003)[36].
Thymosin-α1 (T-α1) is an immune modifier that can trigger maturational events in lymphocytes, augment T-cell function, and promote reconstitution of immune defects[37]. T-α1 has been shown to promote disease remission and cessation of HBV replication in patients with HBeAg-positive chronic hepatitis B without significant side effects[38,39]. Clinical trials using T-α1 in the treatment of patients with immunodeficiency or cancer indicate that this agent is nontoxic, enhances immune responsiveness and augments specific lymphocyte functions, including lymphoproliferative responses to mitogens, maturation of T-cells, antibody production, and T-cell-mediated cytotoxicity[40-42]. On the basis of these observations, we conducted a randomized, controlled trial to compare the efficacy and safety of T-α1 vs INF-α therapy in patients with chronic hepatitis B.
Sixty-two Chinese patients were enrolled in the study. All met the following criteria for entry: age 18-60 years, presence of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) in serum for at least 12 mo, positive serum tests for HBV DNA documented on at least two occasions and at least 3 mo apart 12-mo before entry, aminotransferase levels higher than 1.5 times the upper normal limit for at least 12 mo, and liver biopsy taken within 3 mo before enrollment showing chronic hepatitis. Eligible patients with evidence of cirrhosis were also included. Patients treated with immunosuppressive or antiviral therapy within 1 year before entry, and those with concurrent hepatitis C virus, hepatitis delta virus, and human immunodeficiency virus infections, causes of liver disease other than HBV, intravenous drug abuse, pregnancy, malignancy, chronic renal failure, or other serious medical illness that might interfere with this trial were excluded.
Another 30 patients with the same virological and clinical characteristics, never treated with IFN-α and followed up for at least 12 mo were used as a historical control (HC) group to evaluate the efficacy of the therapies.
The 62 patients were randomly divided into two groups to receive either subcutaneous injection of T-α1 twice a week for 6 mo or of 5 MU IFN-α once a day for 15 d, then 3 times weekly for 6 mo. The patients in HC group were followed up without specific treatment. All patients were assigned to receive general liver-protecting medication in the first month.
Efficacy and safety analysis was performed for all randomized patients who received both thymosin-α1 and interferon-α medication dose in the study. Two predetermined primary measures of efficacy assessed after 24 wk of treatment-free follow-up were HBeAg seroconversion (defined by the loss of HBeAg and the presence of anti-HBe antibody) and suppression of HBV DNA to levels below 1000 copies per milliliter. Secondary efficacy measures assessed after 24 wk of treatment-free follow-up included the combined response (HBeAg seroconversion, normalization of ALT levels, and suppression of HBV DNA levels to below 1000 copies per milliliter). Measures of safety included adverse events, vital signs, hematologic measurements, clinical and chemical measurements, and routine urinalysis. The severity of adverse events was graded on a three-point scale (mild, moderate, and severe), and causality was determined by the investigator. These safety parameters were assessed at wk 0, 1, 2, 4, 6, 8, and every four weeks thereafter throughout the treatment and during the follow-up.
All patients were assessed biweekly for the first 2 mo, and then monthly for 12 mo. Clinical and laboratory assessments consisted of a detailed history, including post injection symptoms and physical examination, routine serum biochemical tests [serum ALT, aspartate transaminase (AST), r-glutamine transpeptidase (r-GT), alkaline phosphatase (AKP), albumin, globulin, bilirubin, etc], complete cell count, markers of HBV replication and urine analysis. All biochemical and hematological tests were performed with routine automated techniques. HBV-markers (HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, and Igm HBcAb) were measured at virological laboratory by using enzyme linked immunosorbent assay (ELISA). Serum HBV DNA was detected by polymerase chain reaction (PCR).
Responses were evaluated both at the end of therapy and at the end of follow-up. A complete virological response was defined as a sustained loss of serum HBeAg in association with the disappearance of serum HBV DNA during the 12-mo study period. A biochemical response was defined as sustained normalization of serum ALT. At the end of treatment and follow-up, a complete response was defined as HBV DNA and HBeAg clearance from the serum and normalization of ALT activity. Relapse was assessed on the basis of ALT flare and/or HBV DNA/HBeAg reappearance during the follow-up period.
Initial calculation was performed with a sample size of 20 patients per treatment group, which provided a statistical power of 80% at the 0.025 level of significance to detect the difference in negative HBV DNA (suppression below 1000 copies per milliliter) rates of 45% vs 6.7% or HBeAg seroconversion rates of 40% vs 3.3%. The sample size increased in 25 patients to allow for withdrawals.
Differences in the final virological and biochemical response rates of two treatment groups and the non-treatment group were compared using the chi square test or Fisher’s exact test when the expected number in the cells was below 5. Odds ratio and corresponding 95% confidence intervals were also computed. P ≤ 0.05 was considered statistically significant.
Of the 92 patients enrolled in the study, 30 were never treated with IFN-α for HBV infection, 2 were non-responders to earlier IFN-α therapy who received T-α1 in the study, 29 patients were randomized to receive T-α1 and 33 to receive IFN-α. All patients completed the 6-mo follow-up period. No significant difference in age, sex, clinical history, biochemical, histological and serological parameters was found among the 3 groups (Table 1).
| Characteristics | HBeAg-positive CHB | ||
| T-α1 group | IFN-α group | HC group | |
| Number | 29 | 33 | 30 |
| Male: Female | 26:3 | 27:6 | 21:9 |
| Age (yr)2 | 45 ± 8 | 42 ± 11 | 44 ± 10 |
| Duration of infection (yr)2 | 10.8 ± 5.6 | 9.3 ± 4.7 | 9.6 ± 5.5 |
| Cirrhosis at entry | 2 | 3 | 2 |
| Previous 1FN therapy | 2 | 0 | 0 |
| Serum ALT (U/L)2 | 177.79 ± 58.82 | 189.94 ± 63.08 | 183.5 ± 68.32 |
| Serum AST (U/L) | 167.72 ± 34.19 | 154.27 ± 73.34 | 141.8 ± 49.85 |
| Albumin (g/L)2 | 44 ± 9 | 47 ± 8 | 45 ± 8 |
| Total bilirubin (μmol/L) | 18.57 ± 9.65 | 17.92 ± 9.22 | 16.68 ± 8.72 |
| Serum HBV DNA (copies/mL)3 | |||
| < 5.0 × 105 copies/mL | 10 (34%) | 15 (45%) | 13 (43%) |
| ≥ 5.0 × 105 copies/mL | 19 (66%) | 18 (55%) | 17 (57%) |
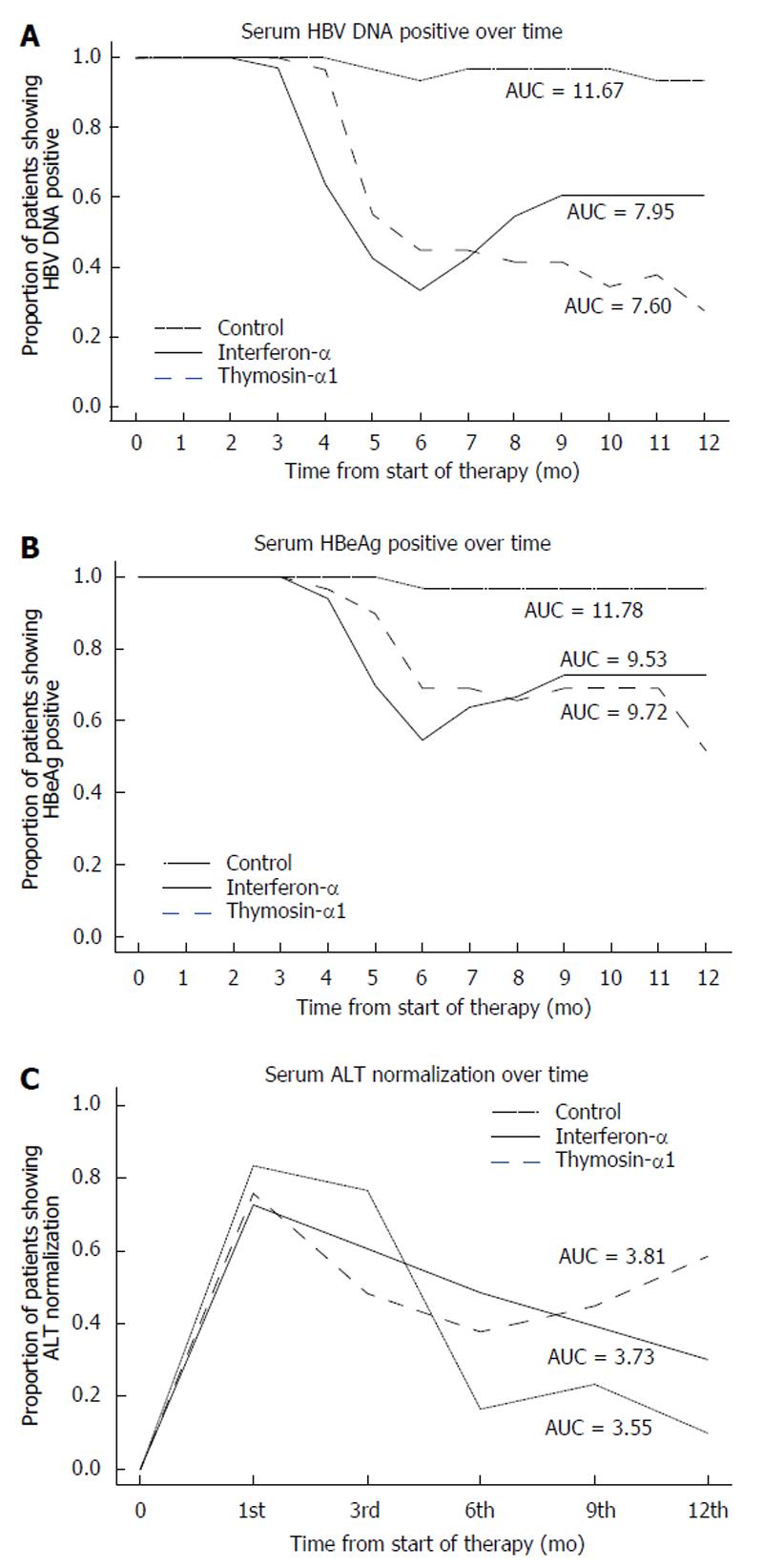
The biochemical and virological modifications at the end of treatment and follow-up period in the two treated groups and the biochemical and virological events in the HC group are listed in Tables 2 and 3 and Figure 1.
At the end of treatment, IFN treatment group had the highest ALT normalization followed by the T-α1 and HC groups, with no statistical significance. The IFN treatment group also had the highest rate of negative HBV DNA and other combined favorable results, differing from those of the HC group but not from those of the T-α1 group. During the follow-up period, the results were however more favorable in the T-α1 group. This group had seven additional patients showing HBV DNA loss (at mo 2-4 in 4 patients and at mo 6 in 3 patients, respectively), whereas HBV DNA reappeared in two patients (at mo 3 and 5, respectively). In the group receiving IFN-α, HBV DNA reappeared in 9 patients (in 3 patients at mo 1, 4 at mo 2 and 2 at mo 3), while no one lost HBV DNA. In the HC group, HBV DNA became negative in 3 of 30 patients (at mo 5, 6, and 11, respectively), whereas HBV DNA reappeared in one patient (at mo 7).
HBV DNA loss was significantly higher in the T-α1 and IFN-α groups than in the HC group at the end of therapy (χ2 = 16.36, P < 0.001 and χ2 = 23.99, P < 0.001, respectively) and follow-up period (χ2 = 26.81, P < 0.001 and χ2 = 9.28, P < 0.01, respectively). The rates of seroconversion of HBeAg antibody in the T-α1, IFN-α and HC groups were 31.0% (9/29), 45.5% (15/33) and 3.3% (1/30), respectively at the end of treatment, and 48.3% (14/29), 27.3% (9/33) and 3.3% (1/30), respectively at the end of follow-up. Serum ALT levels returned to the normal range in 11 of 29 patients given T-α1, in 16 of 33 patients in IFN-α group at the end of treatment and in 5 of 30 of the HC group after 6 mo of follow-up. During the follow-up period, 7 additional patients receiving T-α1 had normal ALT and one patient showed ALT flare, whereas 6 patients of the IFN-α group showed ALT flare, and no one had normal ALT. In the HC group, two patients had normal ALT during months 6 and 12 of follow-up and ALT flare was seen in 4 patients who had normal ALT during the first 6 mo of follow-up. At the end of the study period a complete response (ALT normalization and HBV DNA/HBeAg loss) was observed in 14 of 29 (48.3%) patients treated with T-α1, in 9 of 33 (27.3%) receiving IFN-α, and in 1 of 30 (3.3%) in HC group (T-α1 vs IFN-α and HC, χ2 = 2.93, P > 0.05 and χ2 = 15.71, P < 0.001).
As shown in the graphs (Figure 1), the AUC of HBV DNA and negative HBeAg was 31% and 19% smaller in IFN-α-treated subjects than in HC subjects. The AUC of ALT normalization was 1.5% greater in IFN-α-treated subjects than in HC subjects. The AUC of HBV DNA and negative HBeAg was 34% and 17% smaller in T-α1 treated subjects than in HC subjects, and was 2.2% greater in IFN-α-treated subjects than in HC subjects. The AUC of HBV DNA and negative HBeAg in T-α1 group was 2.9% and 1.6% smaller than in IFN-α-treated subjects.
By the end of the follow-up period, the proportions of ALT normalization and negative HBV DNA in the T-α1 group were significantly higher than those in the IFN-α and HC groups. The odds of ALT normalization and negative HBV DNA at the end of the follow-up was more than three-fold higher in the T-α1 group than in the IFN-α group (Table 4).
Typical side effects of IFN-α treatment, such as flu-like syndrome, fatigue, irritability, and headache, were seen in most of the patients treated with IFN-α. However, no serious or long-term side effects were noted and no patients discontinued the treatment. Therapy with T-α1 was not associated with significant side effects. Three patients reported local discomfort at injection sites. No systemic or constitutional symptoms were observed after T-α1 administration.
The results of the present randomized, controlled trial have shown that T-α1 therapy at a dose of 1.6 mg via subcutaneous injection, twice a week for 6 mo, is effective and safe in patients with chronic hepatitis B. Compared with IFN-α, T-α1 has a slower speed but more sustainable and better end results of virological clearance. Nearly 50% of the treated patients became seronegative for HBeAg and HBV DNA 6 mo after the therapy. This response rate was not only significantly higher than that of the spontaneous seroconversion rate (3.3% in this study), but also obviously higher than the response to IFN-α therapy alone (27.3%) assessed 6 mo after the therapy, suggesting that T-α1 has the same efficacy as IFN-α in inducing clinical and virological remission of chronic hepatitis. The rate of response in terms of ALT normalization and/or HBV DNA and/or HBeAg loss was not significantly different in the T-α1 group compared with the IFN-α group at the end of the treatment (P > 0.05). However, there was a significant difference in the rate of response between the two groups at the end of the follow-up period (P < 0.05). The normalization of serum ALT and loss of HBV DNA and HBeAg was observed more frequently in the IFN-α group at the end of therapy and in the T-α1 group at the end of the follow-up period. Furthermore, the response to the treatment was observed also in the T-α1 group during the follow-up period, but not in the IFN-α group. On the basis of these results and considering that ALT normalization and negative HBV DNA and HBeAg may spontaneously occur in untreated patients, we retrospectively compared the two treated groups with a untreated group which was followed up for at least 12 mo. The results showed that a significant higher rate of complete response occurred in the IFN-α group at the end of therapy and in the T-α1 group at the end of follow-up period than that in the HC group.
The benefit of T-α1 was not immediately significant at the end of therapy and complete virological response had a tendency to increase or accumulate gradually after the therapy in our study. In contrast, the effect of IFN-α was relatively more quick but less sustainable. The beneficial effect of T-α1 has been reported in a multicenter American trial in which 5 of the 12 responders to T-α1 therapy showed a delayed response[43]. This is in contrast to therapy with IFN-α, in which responses usually occur during the first 4 mo of treatment. These contrasting patterns of response have been demonstrated in a recent Italian study involving HBeAg-negative, HBV DNA-positive, interferon-naive patients with a higher ALT level (181 ± 159 U/L), in which the complete response (ALT normalization and HBV DNA loss) rate increased gradually from 29.4% at the end of therapy to 41.2% 6 mo after the T-α1 therapy, the response to IFN therapy decreased from 43.8% at the end of therapy to 25% 6 mo after the therapy[44]. This delayed effect of T-α1 has also been reported by Chien et al[45,46] in patients with chronic hepatitis B recently.
The reasons for the delayed effect of T-α1 are not clear. The delayed response is not likely a result of direct antiviral effects similar to those of interferon. T-α1 may exert an immunoregulatory function that promotes the endogenous antiviral immune response, improves the effectiveness and coordination of the host cellular immune mechanisms in clearing HBV-infected hepatocytes. It was reported that patients treated with T-α1 have a higher peripheral blood helper T cell count (CD4) and IFN- r production by peripheral blood mononuclear cells during and after the T-α1 therapy[39]. In view of the immune mechanisms involved in the pathogenesis of liver injuries in chronic HBV infection, it is possible that T-α1 may activate viral-specific helper T cells, result in the amplification of humoral immune response to viral proteins, induce viral antigen-specific cytotoxic T lymphocytes through secreting endogenous IFN-α, IFN-r, interleukin-2, and tumor necrosis factor, and increase lymphocyte interleukin-2 receptor expression[47-58]. Moreover, T-α1 is able to stimulate natural killer activity by acting synergistically with endogenous IFN-α and IFN-β[41]. Although T-α1 is not known to possess antiviral properties, it was reported that this agent is able to inhibit woodchuck hepatitis virus replication[59,60], suggesting that the delayed effect after T-α1 therapy in the present study is possibly caused by the immunomodulating effect of T-α1 that induces persistently higher helper T-cell function. Because noncytolytic inhibition of HBV RNA, nucleocapsid particles, and replicative DNA intermediates by cytotoxic T lymphocytes has been described in the transgenic mouse model[61,62], it is also possible that viral clearance after T-α1 therapy, particularly preceding ALT flare, is mediated by noncytolytic antiviral effects of cytotoxic T lymphocytes. Further studies are needed to elucidate the possible mechanisms.
In addition to having better HBV clearance, T-α1 is also more tolerable than IFN-α and has no side effects. These together with a small number of weekly injections could favor better patient compliance.
In conclusion, the results of this trial indicate that a 6-mo T-α1 therapy is safe and effective in arresting HBV replication and reducing lobular activity in patients with chronic hepatitis B. Furthermore, T-α1 is better tolerated than IFN-α, and may gradually induce more sustained ALT normalization and HBV DNA/HBeAg loss. A more effective therapeutic approach warrants further study.
The authors acknowledge the cooperation of the staff of the Department of Hepatopathy of Third Kunming Municipal People’s Hospital, the Department of Internal Medicine of Third People’s Hospital of Yunnan Province and Yunnan General Hospital of The Chinese People's Armed Police Forces for participating in the research project. The authors are indebted to the staff of the Epidemiology Unit, Faculty of Medicine, Prince of Songkla University, Thailand, for their critical analysis of the study data, and especially thank Professor Virasakdi Chongsuvivatwong for providing excellent advice and review and technical assistance.
S- Editor Liu Y L- Editor Wang XL E- Editor Bai SH
| 1. | Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493-496. [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 428] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, Pao CC. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology. 1986;90:263-267. [PubMed] [Cited in This Article: ] |
| 3. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2221] [Cited by in F6Publishing: 2124] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 4. | WHO Fact Sheets, available at www. sho.int. Accessed: September 24 2004; . [Cited in This Article: ] |
| 5. | Mahoney FJ. Update on diagnosis, management, and prevention of hepatitis B virus infection. Clin Microbiol Rev. 1999;12:351-366. [PubMed] [Cited in This Article: ] |
| 6. | Malik AH, Lee WM. Chronic hepatitis B virus infection: treatment strategies for the next millennium. Ann Intern Med. 2000;132:723-731. [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 118] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 7. | Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, Liu CJ, Shih WL, Kao JH, Chen DS, Chen CJ. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97:265-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 402] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 8. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2309] [Cited by in F6Publishing: 2210] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 9. | Hanazaki K. Antiviral therapy for chronic hepatitis B: a review. Curr Drug Targets Inflamm Allergy. 2004;3:63-70. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, Gane E, Kao JH, Omata M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 281] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Sánchez-Quijano A, Lissen E. [Treatment of viral hepatitis (I). Treatment of chronic hepatitis B]. Enferm Infecc Microbiol Clin. 2006;24:453-461; quiz 462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Buster EH, Janssen HL. Antiviral treatment for chronic hepatitis B virus infection--immune modulation or viral suppression. Neth J Med. 2006;64:175-185. [PubMed] [Cited in This Article: ] |
| 13. | Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H, Wright TL. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. 2006;4:936-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 14. | Perrillo RP. Interferon in the management of chronic hepatitis B. Dig Dis Sci. 1993;38:577-593. [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Chien RN, Liaw YF. [Drug therapy in patients with chronic type B hepatitis]. J Formos Med Assoc. 1995;94 Suppl 1:S1-S9. [PubMed] [Cited in This Article: ] |
| 16. | Zhu Y, Wang YL, Shi L. Clinical analysis of the efficacy of interferon alpha treatment of hepatitis. World J Gastroenterol. 1998;4 Suppl 2:85-86 Available from: http: //www.wjgnet.com/1007-9327/abstract_en.aspf=tk85b&v=4. [Cited in This Article: ] |
| 17. | Shi JJ, Miao F, Liu FL. Therapeutic effect of medicinal herbs and western drugs on hepatitis B virus. World J Gastroenterol. 1998;4 Suppl 2:61-62 Available from: http: //www.wjgnet.com/1007-9327/abstract_en.aspf=tk61&v=4. [Cited in This Article: ] |
| 18. | Yu YY, Si CW, Tian XL, He Q, Xue HP. Effect of cytokines on liver necrosis. World J Gastroenterol. 1998;4:311-313. [PubMed] [Cited in This Article: ] |
| 19. | Tang ZY, Qi JY, Shen HX, Yang DL, Hao LJ. Short- and Long- term effect of interferon therapy in chronic hepatitis C. China Natl J New Gastroenterol. 1997;3:77 Available from: http: //www.wjgnet.com/1009-3079/5/104.asp. [Cited in This Article: ] |
| 20. | He YW, Liu W, Zen LL, Xiong KJ, Luo DD. Effect of interferon in combination with ribavirin on the plus and minus strand of HCV RNA in patients with chronic hepatitis C. China Natl J New Gastroenterol. 1996;2:179-181 Available from: http: //www.wjgnet.com/1007-9327/2/179.asp. [Cited in This Article: ] |
| 21. | Perrillo RP, Schiff ER, Davis GL, Bodenheimer HC, Lindsay K, Payne J, Dienstag JL, O'Brien C, Tamburro C, Jacobson IM. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med. 1990;323:295-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 639] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Fevery J, Elewaut A, Michielsen P, Nevens F, Van Eyken P, Adler M, Desmet V. Efficacy of interferon alfa-2b with or without prednisone withdrawal in the treatment of chronic viral hepatitis B. A prospective double-blind Belgian-Dutch study. J Hepatol. 1990;11 Suppl 1:S108-S112. [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Krogsgaard K, Bindslev N, Christensen E, Craxi A, Schlichting P, Schalm S, Carreno V, Trepo C, Gerken G, Thomas HC. The treatment effect of alpha interferon in chronic hepatitis B is independent of pre-treatment variables. Results based on individual patient data from 10 clinical controlled trials. European Concerted Action on Viral Hepatitis (Eurohep). J Hepatol. 1994;21:646-655. [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Liaw YF, Lin SM, Chen TJ, Chien RN, Sheen IS, Chu CM. Beneficial effect of prednisolone withdrawal followed by human lymphoblastoid interferon on the treatment of chronic type B hepatitis in Asians: a randomized controlled trial. J Hepatol. 1994;20:175-180. [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1020] [Cited by in F6Publishing: 1066] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 26. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1288] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 27. | Yuen MF, Wong DK, Sum SS, Yuan HJ, Yuen JC, Chan AO, Wong BC, Lai CL. Effect of lamivudine therapy on the serum covalently closed-circular (ccc) DNA of chronic hepatitis B infection. Am J Gastroenterol. 2005;100:1099-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 281] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 521] [Cited by in F6Publishing: 500] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 31. | Liaw YF. Impact of YMDD mutations during lamivudine therapy in patients with chronic hepatitis B. Antivir Chem Chemother. 2001;12 Suppl 1:67-71. [PubMed] [Cited in This Article: ] |
| 32. | Locarnini S, Hatzakis A, Heathcote J, Keeffe EB, Liang TJ, Mutimer D, Pawlotsky JM, Zoulim F. Management of antiviral resistance in patients with chronic hepatitis B. Antivir Ther. 2004;9:679-693. [PubMed] [Cited in This Article: ] |
| 33. | Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 421] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 34. | Ayres A, Bartholomeusz A, Lau G, Lam KC, Lee JY, Locarnini S. Lamivudine and Famciclovir resistant hepatitis B virus associated with fatal hepatic failure. J Clin Virol. 2003;27:111-116. [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1188] [Cited by in F6Publishing: 1098] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 36. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 874] [Cited by in F6Publishing: 802] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 37. | Low TL, Goldstein AL. Thymosins: structure, function and therapeutic applications. Thymus. 1984;6:27-42. [PubMed] [Cited in This Article: ] |
| 38. | You J, Zhuang L, Tang BZ, Yang WB, Ding SY, Li W, Wu RX, Zhang HL, Zhang YM, Yan SM. A randomized controlled clinical trial on the treatment of Thymosin a1 versus interferon-alpha in patients with hepatitis B. World J Gastroenterol. 2001;7:411-414. [PubMed] [Cited in This Article: ] |
| 39. | Mutchnick MG, Appelman HD, Chung HT, Aragona E, Gupta TP, Cummings GD, Waggoner JG, Hoofnagle JH, Shafritz DA. Thymosin treatment of chronic hepatitis B: a placebo-controlled pilot trial. Hepatology. 1991;14:409-415. [DOI] [Cited in This Article: ] |
| 40. | Sztein MB, Goldstein AL. Thymic hormones--a clinical update. Springer Semin Immunopathol. 1986;9:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Goldstein AL, Badamchian M. Thymosins: chemistry and biological properties in health and disease. Expert Opin Biol Ther. 2004;4:559-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Chen C, Li M, Yang H, Chai H, Fisher W, Yao Q. Roles of thymosins in cancers and other organ systems. World J Surg. 2005;29:264-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Mutehnick MG, Lindsay KL, Schiff ER, Cummingo GD, Appelman HD. Thymosin a1 treatment of chronic hepatitis B: a multicenter randomized placebo-controlled double blind study. Gastroenterology. 1995;108:A1127. [DOI] [Cited in This Article: ] |
| 44. | Andreone P, Cursaro C, Gramenzi A, Zavagliz C, Rezakovic I, Altomare E, Severini R, Franzone JS, Albano O, Ideo G. A randomized controlled trial of thymosin-alpha1 versus interferon alfa treatment in patients with hepatitis B e antigen antibody--and hepatitis B virus DNA--positive chronic hepatitis B. Hepatology. 1996;24:774-777. [PubMed] [Cited in This Article: ] |
| 45. | Chien RN, Liaw YF, Chen TC, Yeh CT, Sheen IS. Efficacy of thymosin alpha1 in patients with chronic hepatitis B: a randomized, controlled trial. Hepatology. 1998;27:1383-1387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | You J, Zhuang L, Cheng HY, Yan SM, Qiao YW, Huang JH, Tang BZ, Ma YL, Wu GB, Qu JY. A randomized, controlled, clinical study of thymosin alpha-1 versus interferon-alpha in [corrected] patients with chronic hepatitis B lacking HBeAg in China [corrected]. J Chin Med Assoc. 2005;68:65-72. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Zhou GH, Luo GA, Cao YC, Zhu MS. Study on the quality of recombinant proteins using matrix-assisted laser desorption ionization time of flight mass spectrometry. World J Gastroenterol. 1999;5:235-240. [PubMed] [Cited in This Article: ] |
| 48. | Qian SB, Chen SS. Transduction of human hepatocellular carcinoma cells with human alpha-interferon gene via retroviral vector. World J Gastroenterol. 1998;4:210-213. [PubMed] [Cited in This Article: ] |
| 49. | Tong WB, Zhang CY, Feng BF, Tao QM. Establishment of a nonradioactive assay for 2'-5' oligoadenylate synthetase and its application in chronic hepatitis C patients receiving interferon-alpha. World J Gastroenterol. 1998;4:70-73. [PubMed] [Cited in This Article: ] |
| 50. | Cao GW, Gao J, Du P, QI ZT, Kong XT. Construction of retroviral vectors to induce a strong expression of human class I interferon gene in human hepatocellular carcinoma cells in vitro. China Natl J New Gastroenterol. 1997;3:139-142 Available from: http: //www.wjgnet.com/1007-9327/3/139.asp. [Cited in This Article: ] |
| 51. | He YW, Liu W, Zen LL, Luo DD. Effects of r interferon on hepatic fibrosis of schistosoma japonicum infected mice. China Natl J New Gastroenterol. 1997;3:6-8 Available from: http: //www.wjgnet.com/1007-9327/3/6.asp. [Cited in This Article: ] |
| 52. | Chen SB, Miao XH, Du P, Wu QX. Assessment of natural and interleukin 2-induced production of interferon gamma in patients with liver diseases. China Natl J New Gastroenterol. 1996;2:173-175 Available from: http: //www.wjgnet.com/1007-9327/2/173.asp. [Cited in This Article: ] |
| 53. | Huang CF, Lin SS, Ho YC, Chen FL, Yang CC. The immune response induced by hepatitis B virus principal antigens. Cell Mol Immunol. 2006;3:97-106. [PubMed] [Cited in This Article: ] |
| 54. | De Carli M, D'Elios MM, Zancuoghi G, Romagnani S, Del Prete G. Human Th1 and Th2 cells: functional properties, regulation of development and role in autoimmunity. Autoimmunity. 1994;18:301-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Tsai SL, Chen MH, Yeh CT, Chu CM, Lin AN, Chiou FH, Chang TH, Liaw YF. Purification and characterization of a naturally processed hepatitis B virus peptide recognized by CD8+ cytotoxic T lymphocytes. J Clin Invest. 1996;97:577-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Marinos G, Torre F, Chokshi S, Hussain M, Clarke BE, Rowlands DJ, Eddleston AL, Naoumov NV, Williams R. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology. 1995;22:1040-1049. [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Milich DR. Influence of T-helper cell subsets and crossregulation in hepatitis B virus infection. J Viral Hepat. 1997;4 Suppl 2:48-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Tsai SL, Sheen IS, Chien RN, Chu CM, Huang HC, Chuang YL, Lee TH, Liao SK, Lin CL, Kuo GC. Activation of Th1 immunity is a common immune mechanism for the successful treatment of hepatitis B and C: tetramer assay and therapeutic implications. J Biomed Sci. 2003;10:120-135. [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Gerin JL, Korba BE, Cote PJ, Tennant BC. A preliminary report of a controlled study of thymosin alpha-1 in the woodchuck model of hepadnavirus infection. Adv Exp Med Biol. 1992;312:121-123. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Menne S, Maschke J, Tolle TK, Lu M, Roggendorf M. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J Virol. 1997;71:65-74. [PubMed] [Cited in This Article: ] |
| 61. | Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25-36. [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 820] [Article Influence: 29.3] [Reference Citation Analysis (1)] |
| 62. | Tsui LV, Guidotti LG, Ishikawa T, Chisari FV. Posttra-nscriptional clearance of hepatitis B virus RNA by cytotoxic T lymphocyte-activated hepatocytes. Proc Natl Acad Sci USA. 1995;92:12398-12402. [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 97] [Article Influence: 3.3] [Reference Citation Analysis (0)] |