Published online Oct 21, 2006. doi: 10.3748/wjg.v12.i39.6261
Revised: May 28, 2006
Accepted: June 14, 2006
Published online: October 21, 2006
In recent years, the results of several studies suggest that human liver tumors can be derived from hepatic progenitor cells rather than from mature cell types. The available data indeed strongly suggest that most combined hepatocellular-cholangiocarcinomas arise from hepatic progenitor cells that retained their potential to differentiate into the hepatocytic and biliary lineages. Hepatic progenitor cells could also be the basis for some hepatocellular carcinomas and hepatocellular adenomas, although it is very difficult to determine the origin of an individual hepatocellular carcinoma. There is currently not enough data to make statements regarding a hepatic progenitor cell origin of cholangiocarcinoma. The presence of hepatic progenitor cell markers and the presence and extent of the cholangiocellular component are factors that are related to the prognosis of hepatocellular carcinomas and combined hepatocellular-cholangiocarcinomas, respectively.
- Citation: Libbrecht L. Hepatic progenitor cells in human liver tumor development. World J Gastroenterol 2006; 12(39): 6261-6265
- URL: https://www.wjgnet.com/1007-9327/full/v12/i39/6261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i39.6261
The traditional view that adult human liver tumors arise from mature cell types has been challenged in recent decades. The results of several studies suggest that some types of human liver tumors can be derived from hepatic progenitor cells (HPCs). In this review, the evidence for a HPC origin of several types of liver tumors and the possible clinical implications are discussed.
HPCs are small epithelial cells with an oval nucleus, scant cytoplasm and location in the bile ductules and canals of Hering[1]. HPCs can differentiate towards the biliary and hepatocytic lineages. Differentiation towards the biliary lineage occurs via formation of reactive bile ductules, which are anastomosing ductules lined by immature biliary cells with a relatively large and oval nucleus surrounded by a small rim of cytoplasm. Hepatocytic differentiation leads to the formation of intermediate hepatocyte-like cells, which are defined as polygonal cells with a size intermediate between that of HPCs and hepatocytes[1]. In most liver diseases, hepatic progenitor cells are “activated”, which means that they proliferate and differentiate towards the hepatocytic and/or biliary lineages. The extent of activation is correlated with disease severity[2-4].
HPCs and their immediate biliary and hepatocytic progeny not only have a distinct morphology, but they also express several markers, with many also present in bile duct epithelial cells[1,5]. Immunohistochemistry using antibodies against these markers facilitates the detection of HPCs. The most commonly used markers are cytokeratin (CK) 19 and CK7[1,6].
The proposal that a human hepatocellular carcinoma (HCC) does not necessarily arise from a mature hepatocyte, but could have a HPC origin, has classically been based on three different types of observations. Each of them, however, gives only indirect evidence that can be disputed.
Firstly, it has been shown that HPCs are the cells of origin of HCC in some animal models of hepatocarcinogenesis[7,8], which has led to the suggestion that this might also be the case in humans. However, in other animal models, the HCCs arise form mature hepatocytes and not from HPCs or reactive bile ductular cells[9,10]. Since it is currently insufficiently clear which of these animal models accurately mimics human hepatocarcinogenesis[11,12], one should be careful about extrapolating data regarding the HPC origin of HCC in animal models to the human situation.
Secondly, liver diseases that are characterized by the presence of carcinogens and development of dysplastic lesions also show HPC activation[1]. Therefore, the suggestion has been made that HPCs form a “target population” for carcinogens[3,13], but this is only a theoretical possibility not supported by experimental data.
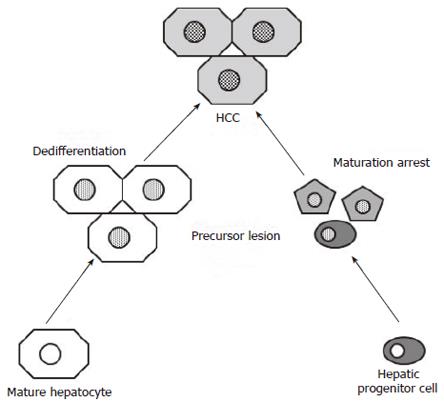
Thirdly, several studies have shown that a considerable proportion of HCCs express one or more HPC markers that are not present in normal, mature hepatocytes[14-18]. Due to the fact that most HPC markers are also expressed in the biliary lineage, the term “biliary marker” has been used in some of these studies[16,17]. The “maturation arrest” hypothesis[19] states that genetic alterations occurring in a HPC, or its immediate progeny, cause aberrant proliferation and prevent its normal differentiation (Figure 1). Further accumulation of genetic alterations eventually leads to malignant transformation of these incompletely differentiated cells. The resulting HCC expresses HPC markers as evidence of its origin. However, expression of HPC markers can also be interpreted in the setting of the “dedifferentiation” hypothesis[19], which suggests that the expression of HPC markers is acquired during tumor progression as a consequence of accumulating mutations (Figure 1). For example, experiments in which human HCC cell lines were transplanted into nude mice have nicely shown that the expression of the HPC marker, CK19, steadily increased when the tumors became increasingly aggressive and metastasized to the lungs[20]. Thus, the expression of CK19 in a HCC does not necessarily mean that the tumor has a HPC origin, but it can also be a mutation-induced, acquired expression associated with tumor progression. Both possibilities are not mutually exclusive.
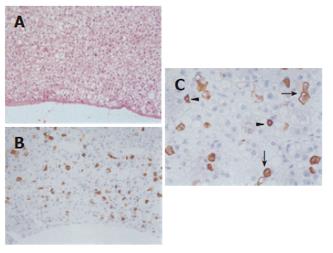
To further address this issue, precursor lesions of HCCs have been investigated for the presence of HPCs and their immediate hepatocytic progeny. It was found that HPCs and intermediate hepatocyte-like cells were present in 50% of small cell dysplastic foci and hepatocellular adenomas[21,22]. Since both types of these lesions are precursors of HCC[12], this supports the idea that at least some HCCs are HPC-derived. Indeed, if the expression of HPC markers in HCCs would always be acquired during tumoral progression, these precursor lesions would not contain HPCs and their immediate hepatocytic progeny (Figure 1). Furthermore, HPCs and intermediate hepatocyte-like cells in hepatocellular adenomas were present in a “starry sky” pattern on a background of mature hepatocytes (Figure 2)[22]. This picture is in agreement with the “cancer stem cell” theory: stem cells that have undergone transformation retain their renewal properties and nourish the tumor by their continuous proliferation and differentiation[23]. Hepatocellular adenomas with HPCs and intermediate hepatocyte-like cells did not form a specific clinicopathological subtype[22].
Although these findings further support a HPC origin for some human HCCs, they certainly do not give definite proof. Moreover, these are findings and hypotheses that apply to HCC in general. For an individual HCC that expresses a HPC marker, it remains impossible to determine whether this marker reflects the cellular origin and/or is caused by tumor progression. This can only be elucidated by determining whether the HCC contains cells that are ultrastructurally identical to HPCs in non-tumoral liver. Until now, this type of investigation has only been done for some hepatocellular adenomas and a very small number of HCCs[22,24].
From a purely clinical viewpoint, the reason why some HCCs express HPC markers is not important. However, the fact that several studies have shown that HCCs expressing HPC markers have a worse prognosis than HPC marker-negative HCCs might be potentially clinically relevant[14-17]. All but one[14] of these studies also reported that HPC marker-positive HCCs were less differentiated than marker-negative HCCs. Multivariate analysis was performed in only one of these studies and expression of the HPC marker CK19 was an independent predictor of postoperative recurrence[15]. This finding needs to be confirmed before one can consider using CK19-expression for prognostication instead of more strongly established independent prognostic markers, such as the immunohistochemical expression of the cell proliferation marker Ki-67[25-27].
In a recent study, unsupervised clustering of microarray data from human HCCs and rat hepatoblasts led to the identification of a subgroup of HCCs that resembled rat hepatoblasts, designated “hepatoblast (HB) subtype” of HCC[11]. This HB subtype of HCCs was characterized by the high expression of several HPC markers, such as CK7 and CK19. However, the expression of other known HPC markers, such as alpha-fetoprotein and ABCB1[28,29], was not different and was even lower, respectively, in HB subtype HCCs when compared to the “hepatocyte subtype” of HCCs. Therefore, the exact relationship between HPCs and this HB subtype of HCCs remains unclear. Remarkably, HB subtype HCCs consisted of tumors associated with a very poor prognosis and the HB signature had a very strong and independent prognostic power. Unfortunately, direct application in clinical practice will not be possible because microarrays can not be performed in every hospital and, more importantly, the method relies on unsupervised clustering. This means that adding new HCCs to the group most likely will alter the dendrogram and hence also the subtype of several of the already included HCCs. This effect has already been observed in breast cancer[30].
Three subtypes of combined hepatocellular-cholangiocarcinoma (CHC) can be distinguished. The first type consists of the so-called “collision tumors” and “double cancers” in which the HCC and the cholangiocarcinoma components are either completely separated or sharply demarcated from each other, indicating that these tumors arise from the coincidental occurrence (and collision) of a HCC and a cholangiocarcinoma[31,32]. The possible HPC origin of the independent components are discussed in the sections on HCC and cholangiocarcinoma.
The second type of CHC represents tumors containing unequivocal (i.e. recognizable on a hematoxylin-eosin-stained section) hepatocellular and cholangiocellular components that are intimately admixed[33,34]. In almost all cases, these tumors also show a variable amount of so-called “transitional areas” that consist of immature-appearing cells that have morphological and immunohistochemical features of both hepatocytes and cholangiocytes[32,33,35]. The cells in the transitional area sometimes resemble HPCs and reactive bile ductules in the non-tumoral liver[33,35-37]. Overall, this picture strongly suggests that the origin of these tumors lies in a HPC that has undergone malignant transformation while differentiating in both the hepatocytic and biliary lineages. It is not completely excludable that the cholangiocellular components are directly derived from the hepatocellular components or vice versa, but this can not account for the presence of tumor cells with morphological, immunohistochemical and architectural features reminiscent of HPCs and reactive bile ductules.
CHCs that consist (almost) completely of such transitional areas and contain only very limited or no unequivocal hepatocellular and cholangiocellular components have been designated as “primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype” and they are thus also likely to have a HPC origin[37,38]. However, only a few cases of this type of tumor have been described, so it is currently not clear whether this third CHC subtype has clinical and prognostic features that are distinct from the second subtype of CHCs.
In contrast, there exist several rather large studies (i.e. at least 20 patients) on the clinicopathological features of the second type of CHCs, including a comparison with HCC and cholangiocarinoma[35,39-42]. Although the results of these studies are conflicting regarding the associations with cirrhosis and hepatitis B or C infection, there is a general consensus that these CHCs are almost always invasive and have a prognosis that lies in between that of HCC and cholangiocarcinoma. A recent study has nicely revealed that CHCs with an extensive cholangiocellular component are more frequently invasive and metastatic to lymph nodes and have a worse prognosis than those with a limited cholangiocellular component[35]. So, it is the extent of differentiation towards the biliary lineage rather than the HPC origin as such that determines the specific prognostic features of this type of tumor.
The issue of whether some cholangiocarcinomas could arise from HPCs rather than from mature cholangiocytes has not yet been investigated in detail. It has been shown that cholangiocarcinoma can originate from HPCs in some animal models[43,44] and a considerable proportion of human cholangiocarcinomas express the neural cell adhesion molecule, which is a marker for reactive bile ductules not present in mature cholangiocytes[45]. However, more studies are needed before statements regarding the HPC origin of cholangiocarcinoma can be made.
The available data strongly suggest that most CHCs arise from HPCs. Furthermore, HPCs could also be the basis of some HCCs and hepatocellular adenomas, although it is very difficult to determine the origin of a specific, individual HCC. There is currently not enough data to make statements regarding a HPC origin of cholangiocarcinoma. The presence of HPC markers and the presence and extent of the cholangiocellular component are factors that are related to the prognosis of HCCs and CHCs, respectively.
Figure 2 is reprinted with permission from: Libbrecht et al. Hepatic progenitor cells in hepatocellular adenomas. Am J Surg Pathol 25: 1388.
S- Editor Wang J L- Editor Lutze M E- Editor Bai SH
| 1. | Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002;13:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 332] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Libbrecht L, Desmet V, Van Damme B, Roskams T. Deep intralobular extension of human hepatic 'progenitor cells' correlates with parenchymal inflammation in chronic viral hepatitis: can 'progenitor cells' migrate. J Pathol. 2000;192:373-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 4. | Eleazar JA, Memeo L, Jhang JS, Mansukhani MM, Chin S, Park SM, Lefkowitch JH, Bhagat G. Progenitor cell expansion: an important source of hepatocyte regeneration in chronic hepatitis. J Hepatol. 2004;41:983-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Alison MR, Lovell MJ. Liver cancer: the role of stem cells. Cell Prolif. 2005;38:407-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Tan J, Hytiroglou P, Wieczorek R, Park YN, Thung SN, Arias B, Theise ND. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver. 2002;22:365-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Dumble ML, Croager EJ, Yeoh GC, Quail EA. Generation and characterization of p53 null transformed hepatic progenitor cells: oval cells give rise to hepatocellular carcinoma. Carcinogenesis. 2002;23:435-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Braun L, Mikumo R, Fausto N. Production of hepatocellular carcinoma by oval cells: cell cycle expression of c-myc and p53 at different stages of oval cell transformation. Cancer Res. 1989;49:1554-1561. [PubMed] [Cited in This Article: ] |
| 9. | Bralet MP, Pichard V, Ferry N. Demonstration of direct lineage between hepatocytes and hepatocellular carcinoma in diethylnitrosamine-treated rats. Hepatology. 2002;36:623-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Lin YZ, Brunt EM, Bowling W, Hafenrichter DG, Kennedy SC, Flye MW, Ponder KP. Ras-transduced diethylnitrosamine-treated hepatocytes develop into cancers of mixed phenotype in vivo. Cancer Res. 1995;55:5242-5250. [PubMed] [Cited in This Article: ] |
| 11. | Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 729] [Cited by in F6Publishing: 719] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 12. | Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int. 2005;25:16-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Hsia CC, Thorgeirsson SS, Tabor E. Expression of hepatitis B surface and core antigens and transforming growth factor-alpha in "oval cells" of the liver in patients with hepatocellular carcinoma. J Med Virol. 1994;43:216-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, Tanaka S, Kaneda K, Hirohashi K. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci. 2003;94:851-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Yoon DS, Jeong J, Park YN, Kim KS, Kwon SW, Chi HS, Park C, Kim BR. Expression of biliary antigen and its clinical significance in hepatocellular carcinoma. Yonsei Med J. 1999;40:472-477. [PubMed] [Cited in This Article: ] |
| 17. | Wu PC, Fang JW, Lau VK, Lai CL, Lo CK, Lau JY. Classification of hepatocellular carcinoma according to hepatocellular and biliary differentiation markers. Clinical and biological implications. Am J Pathol. 1996;149:1167-1175. [PubMed] [Cited in This Article: ] |
| 18. | Van Eyken P, Sciot R, Paterson A, Callea F, Kew MC, Desmet VJ. Cytokeratin expression in hepatocellular carcinoma: an immunohistochemical study. Hum Pathol. 1988;19:562-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 99] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Sell S. Cellular origin of cancer: dedifferentiation or stem cell maturation arrest. Environ Health Perspect. 1993;101 Suppl 5:15-26. [PubMed] [Cited in This Article: ] |
| 20. | Ding SJ, Li Y, Tan YX, Jiang MR, Tian B, Liu YK, Shao XX, Ye SL, Wu JR, Zeng R. From proteomic analysis to clinical significance: overexpression of cytokeratin 19 correlates with hepatocellular carcinoma metastasis. Mol Cell Proteomics. 2004;3:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Libbrecht L, Desmet V, Van Damme B, Roskams T. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol. 2000;33:76-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Libbrecht L, De Vos R, Cassiman D, Desmet V, Aerts R, Roskams T. Hepatic progenitor cells in hepatocellular adenomas. Am J Surg Pathol. 2001;25:1388-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 851] [Cited by in F6Publishing: 826] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 24. | Xiao JC, Ruck P, Kaiserling E. Small epithelial cells and the histogenesis of hepatocellular carcinoma. Morphological, immunohistochemical and electron miroscopical findings. Pathol Res Pract. 1996;192:343. [Cited in This Article: ] |
| 25. | Fiorentino M, Altimari A, Ravaioli M, Gruppioni E, Gabusi E, Corti B, Vivarelli M, Bringuier PP, Scoazec JY, Grigioni WF. Predictive value of biological markers for hepatocellular carcinoma patients treated with orthotopic liver transplantation. Clin Cancer Res. 2004;10:1789-1795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Guzman G, Alagiozian-Angelova V, Layden-Almer JE, Layden TJ, Testa G, Benedetti E, Kajdacsy-Balla A, Cotler SJ. p53, Ki-67, and serum alpha feto-protein as predictors of hepatocellular carcinoma recurrence in liver transplant patients. Mod Pathol. 2005;18:1498-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Ito Y, Matsuura N, Sakon M, Takeda T, Umeshita K, Nagano H, Nakamori S, Dono K, Tsujimoto M, Nakahara M. Both cell proliferation and apoptosis significantly predict shortened disease-free survival in hepatocellular carcinoma. Br J Cancer. 1999;81:747-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J Pathol. 2003;200:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Bisgaard HC, Holmskov U, Santoni-Rugiu E, Nagy P, Nielsen O, Ott P, Hage E, Dalhoff K, Rasmussen LJ, Tygstrup N. Heterogeneity of ductular reactions in adult rat and human liver revealed by novel expression of deleted in malignant brain tumor 1. Am J Pathol. 2002;161:1187-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Chang JC, Hilsenbeck SG, Fuqua SA. Genomic approaches in the management and treatment of breast cancer. Br J Cancer. 2005;92:618-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Haratake J, Hashimoto H. An immunohistochemical analysis of 13 cases with combined hepatocellular and cholangiocellular carcinoma. Liver. 1995;15:9-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 33. | Tickoo SK, Zee SY, Obiekwe S, Xiao H, Koea J, Robiou C, Blumgart LH, Jarnagin W, Ladanyi M, Klimstra DS. Combined hepatocellular-cholangiocarcinoma: a histopathologic, immunohistochemical, and in situ hybridization study. Am J Surg Pathol. 2002;26:989-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Wittekind C, Fisher HP, Ponchon T. Combined hepatocellular and cholangiocarcinoma. Lyon: IARC Press 2000; 181. [Cited in This Article: ] |
| 35. | Aishima S, Kuroda Y, Asayama Y, Taguchi K, Nishihara Y, Taketomi A, Tsuneyoshi M. Prognostic impact of cholangiocellular and sarcomatous components in combined hepatocellular and cholangiocarcinoma. Hum Pathol. 2006;37:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Tanaka S, Yamamoto T, Tanaka H, Kodai S, Ogawa M, Ichikawa T, Hai S, Sakabe K, Uenishi T, Shuto T. Potentiality of combined hepatocellular and intrahepatic cholangiocellular carcinoma originating from a hepatic precursor cell: Immunohistochemical evidence. Hepatol Res. 2005;32:52-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 38. | Robrechts C, De Vos R, Van den Heuvel M, Van Cutsem E, Van Damme B, Desmet V, Roskams T. Primary liver tumour of intermediate (hepatocyte-bile duct cell) phenotype: a progenitor cell tumour. Liver. 1998;18:288-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, Ojima H, Sakamoto M, Takayama T, Makuuchi M. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003;33:283-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 244] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 42. | Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998;13:34-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Lee JH, Rim HJ, Sell S. Heterogeneity of the "oval-cell" response in the hamster liver during cholangiocarcinogenesis following Clonorchis sinensis infection and dimethylnitrosamine treatment. J Hepatol. 1997;26:1313-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Steinberg P, Steinbrecher R, Radaeva S, Schirmacher P, Dienes HP, Oesch F, Bannasch P. Oval cell lines OC/CDE 6 and OC/CDE 22 give rise to cholangio-cellular and undifferentiated carcinomas after transformation. Lab Invest. 1994;71:700-709. [PubMed] [Cited in This Article: ] |
| 45. | Asayama Y, Aishima S, Taguchi K, Sugimachi K, Matsuura S, Masuda K, Tsuneyoshi M. Coexpression of neural cell adhesion molecules and bcl-2 in intrahepatic cholangiocarcinoma originated from viral hepatitis: relationship to atypical reactive bile ductule. Pathol Int. 2002;52:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |