Published online Sep 21, 2006. doi: 10.3748/wjg.v12.i35.5599
Revised: July 10, 2006
Accepted: July 18, 2006
Published online: September 21, 2006
H pylori is probably the most prevalent human pathogen worldwide. Since it was initially suggested in 1983 by Marshall and Warren to be implicated in gastritis and peptic ulcer disease, H pylori has also been implicated in gastric carcinoma and was classified as a class I carcinogen. In the last two decades, a noteworthy body of research has revealed the multiple processes that this gram negative bacterium activates to cause gastroduodenal disease in humans. Most infections are acquired early in life and may persist for the life of the individual. While infected individuals mount an inflammatory response that becomes chronic, along with a detectable adaptive immune response, these responses are ineffective in clearing the infection. H pylori has unique features that allow it to reside within the harsh conditions of the gastric environment, and also to evade the host immune response. In this review, we discuss the various virulence factors expressed by this bacterium and how they interact with the host epithelium to influence pathogenesis.
-
Citation: Beswick EJ, Suarez G, Reyes VE.
H pylori and host interactions that influence pathogenesis. World J Gastroenterol 2006; 12(35): 5599-5605 - URL: https://www.wjgnet.com/1007-9327/full/v12/i35/5599.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i35.5599
H pylori is one of the most common pathogens affecting humankind, infecting approximately 50% of the world’s population. This pathogen is a gram-negative spiral shaped bacterium that has the unique ability to colonize the human gastric mucosa. The infection is usually acquired early in life and may persist a lifetime, unless treated. Of those infected, many will develop asymptomatic gastritis, but 10% develop gastric or duodenal ulcers, and approximately 1% develop gastric carcinoma. The outcome of the infection may involve a combination of the bacterial factors, host factors, as well as environmental factors. Ulceration and carcinogenesis are mutually exclusive outcomes of this infection. H pylori infection is a very persistent infection, and in areas of high prevalence, reinfection is also very common.
A very high percentage of gastric and duodenal ulcers (up to 85%) are attributable to H pylori infection. Patients in the United States who are infected with H pylori have a 3.5 times increased risk of developing peptic ulcer disease than uninfected persons[1]. A hallmark feature of infection with H pylori is a pronounced inflammatory response and the inability of the host to clear the infection, which results in a persistent infection, increased acid production, and tissue damage.
It is now well accepted that chronic infection with H pylori is a major risk factor in the development of gastric cancer. H pylori has been shown to induce changes in the gastric mucosa that could contribute to the development of cancer. Given the strength of the evidence supporting an association between adenocarcinomas of the gastric mucosa and H pylori infection, H pylori has become classified as a class I carcinogen by the International Agency for Research on Cancer in affiliation with the World Health Organization[2]. Gastric cancer remains the second deadliest cancer worldwide. On a global scale, gastric cancer accounts for approximately 700 000 deaths annually. In the US there are 24 000 new cases and 14 000 deaths annually[3].
Infection with H pylori also plays a critical role in the development of mucosa-associated lymphoid tissue (MALT) lymphoma. H pylori is present in the gastric mucosa of most cases of MALT lymphoma, and 75% of these cases regress after eradication of H pylori[4,5]. Interestingly, gastric MALT lymphoma is the only known malignancy whose course can be directly changed by the removal of a pathogen. Thus, H pylori-associated diseases are a significant global problem and result in considerable morbidity, mortality, and societal costs.
H pylori colonizes the gastric epithelial apical surface, but the precise mechanisms of adherence and pathogenesis are still being elucidated. Adherent strains are able to survive in the gastric mucosa, colonize at high densities, and are able to re-colonize, while non-adherent strains are readily removed[6]. Thus, adhesion is crucial in the ability of H pylori to persist and cause disease. In addition to contributing to colonization, adherence results in signal transduction, activation of NF-κB and subsequent secretion of interleukin-8, which is important in the inflammatory response during infection.
An assortment of molecules on gastric epithelial cells (GECs) have been proposed as receptors for H pylori adherence, as well as multiple adhesins that have been identified on the outer membrane of H pylori, but those responsible for pathogenic events are still being investigated (Table 1). Several well known adhesins are BabA, SabA, and AlpAB. BabA and SabA bind to fucosylated and sialylated blood group antigens, respectively. There are clearly multiple adhesins and receptors for H pylori because only half of the strains in the U.S. have detectable BabA[7]. While the attachment of H pylori using BabA as an adhesin does not appear to induce signaling or immune responses from host cells, SabA appears to be required for activation of neutrophils and the resulting oxidative burst by binding to sialylated neutrophil receptors[8]. Although the AlpAB receptor is unknown, it may be even more important as an adhesin because studies with knockout strains dramatically reduced adherence of the bacteria to some cells[9]. HopZ, another adhesin being investigated, also showed decreased adherence when a knockout strain was utilized[10], but not as dramatically as the AlpAB knockout strain. H pylori urease can also act as a bacterial adhesin[11]. Urease present on the bacterial surface due to bacterial lysis or release[12,13] binds to class II MHC molecules on host cells, and may induce their apoptosis[11].
| Adhesin | Receptor |
| BabA | Lewis B blood group antigen |
| SabA/B | Sialyl Lewis X |
| HpaA | Sialyl Lewis X |
| UreB | CD74 |
| UreA? | Class II MHC |
| AlpA/B | ? |
| HopZ | ? |
| ? | DAF |
| ? | Sulfated molecules (heparan sulfate) |
| ? | Phospholipids |
| ? | Trefoil Factor 1 |
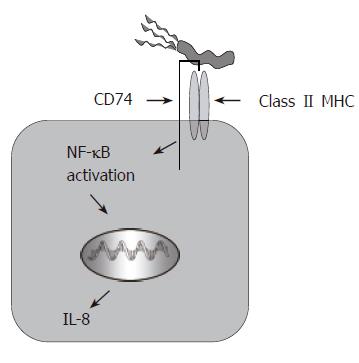
Other studies from this group also suggest that the urease B subunit binds to CD74, which is expressed in polarized fashion on the luminal side of the epithelium[14], and in doing so stimulates gastric epithelial IL-8 release[15] (Figure 1). A recent study by O’Brien and colleagues showed that, similar to other pathogens, H pylori uses decay accelerating factor (DAF aka CD55) as a receptor for binding to the gastric epithelium[16]. Since there have been multiple H pylori adhesins described, bacterial adhesion is clearly a complex mechanism with multiple outcomes depending on the host cell receptor engaged.
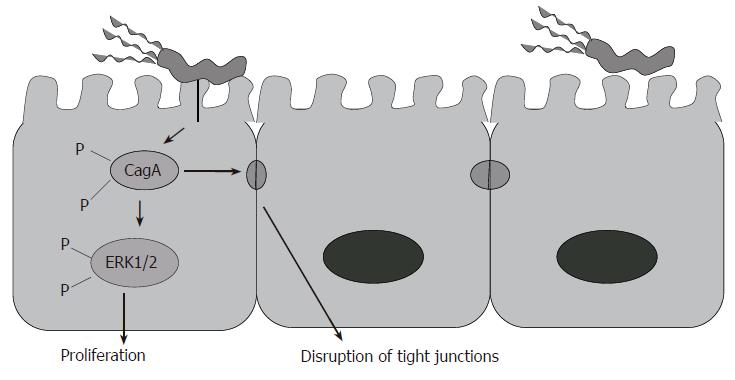
Following colonization and attachment, various virulence factors expressed by certain H pylori strains appear to promote disease. For instance, the expression of cagA and vacA genes by strains of H pylori is highly associated with disease[17]. These cagA gene-expressing strains have also been associated with peptic ulcer and patients infected with these strains have an increased risk of gastric cancer[18]. The cagA gene is considered a marker for a cluster of genes referred to as pathogenicity island (PAI). cagPAI is known to encode for a type IV secretion system that allows CagA, and possibly peptidoglycan, to be delivered into epithelial cells (Figure 2). CagA is tyrosine phosphorylated by Src family kinases[19], and has differing numbers of tyrosine phosphorylation motifs (EPIYA motifs), which determine the virulence of the H pylori strain and host cell response to it. The amount of EPIYA motifs is directly related to the levels of phosphorylation and cytoskeletal rearrangement seen in epithelial cells[20]. Phosphorylated CagA interacts with a protein tyrosine phosphatase, SHP-2 inducing its phosphatase activity. Upon activation of SHP-2, it is able to induce host cells signaling, such as MAP kinase/MEK/ERK1/2 signaling through Ras/Raf. Dysregulation in this pathway is responsible for increased cell proliferation and moving of gastric epithelial cells (cell spreading) and cell elongation (hummingbird phenotype)[21]. Interaction of CagA with other signaling molecules such as growth factor receptor-binding protein- 2 (Grb-2), hepatocyte growth factor scatter factor receptor (c-Met), and phospholipase C gamma (PLC-γ) can induce similar phenotypes in gastric epithelial cells[22,23]. Phosphorylated CagA inhibits the activity of Src kinases in a negative feedback loop[19]. Thus, the inhibition on Src kinases activity also results in dephosphorylation of a set of host cell proteins, including the actin-binding protein cortactin. In parallel to ERK1/2 signaling, NF-κB may also be activated, and upregulation of pro-inflammatory cytokines may also ensue[24]. While it was initially thought that the CagA injection was not responsible for the production of proinflammatory cytokines, but other proteins of the cagPAI were thought to play a crucial role, it has more recently been shown that CagA injection into the host gastric epithelial cell can induce NF-κB activation and IL-8 production[24]. In fact, independent studies showed that transfection of cagA into gastric epithelial cells induced IL-8 production[25]. The activation of the Ras/MEK/ERK pathway occurs following the interaction of CagA with Grb2. Because CagA interacts with important signaling mediators in the host cells, it is considered responsible for changes in cell morphology, adhesion and turnover. CagA also induces the recruitment of ZO-1 and junctional adhesion molecule (JAM) to the site of bacterial attachment causing disruption of the epithelial barrier and dysplastic alterations in epithelial cell morphology. This effect is phosphorylation independent[26]. The delivery of H pylori peptidoglycan via the cagPAI-encoded type IV secretion system results in the intracellular binding of peptidoglycan by Nod1, an intracellular pathogen-recognition molecule, and this may also contribute to the induction of gastric epithelial responses[27]. The H pylori Type IV secretion system (T4SS) stimulates the Rho family GTPases Rac1 and Cdc42 in gastric epithelial cells in an independent CagA translocation mechanism[28]. Thus, Rac1 and Cdc42 are recruited to the membrane at sites of infection activating p21-activated kinase (PAK1) and rearrangements of the cytoskeleton[29]. The T4SS can also activate the receptor tyrosine kinase, epidermal growth factor receptor (EGFR), regulating the ERK1/2 pathway via Ras phosphorylation[30]. Furthermore, CagA can inhibit B-cell proliferation by suppressing the JAK-STAT signaling pathway. Hence CagA, in a phosphorylation-independent pathway, can diminish the immune response against H pylori and play a role in the development of MALT lymphoma by impairing p53-dependent apoptosis pathway[31].
The vacuolating toxin, or VacA, is a pore forming toxin that has the ability to induce vacuole formation in cells and disrupt normal membrane trafficking. VacA, is expressed by about half of all H pylori strains. Like CagA, VacA appears to be unique to H pylori since no other species have a homologue. VacA has effects on many cell types, including gastric epithelial cells, antigen presenting cells, mast cells, and lymphocytes, which makes it an important virulence factor. This toxin is secreted by H pylori, and it binds to the plasma membrane of host cells where it forms anion-selective channels. The receptors for VacA are EGFR and RPTP-α and -β[32]. However, VacA also binds to detergent-resistant microdomains (lipid rafts) and GPI-APs[33,34]. This process results in the release of nutrients from the cell, and the bacteria may use these for survival[35]. As the anion concentration becomes higher inside the cell through these pores, proton pumping also increases, as does an influx of weak bases. The weak bases are protonated and trapped inside, causing osmotic swelling, and the formation of a vacuole[36]. VacA can also disrupt mitochondrial membrane potential and affect cellular ATP concentrations, which disrupt the cell cycle progression and lead to apoptosis[37,38]. Another significant way in which VacA contributes to pathogenesis is by inhibiting the processing of antigens by B-cells and their presentation to CD4+ T-cells[39], as well as the T cell activation and proliferation. When mixed with T cells, VacA supresses NFAT[40,41,42], IL-2 production, and surface expression of IL-2 receptors, which are required for T cell proliferation and viability[36]. Multiple signaling pathways of T cell activation are also affected by VacA exposure, which is one mechanism H pylori may use to evade immune responses. In gastric epithelial cells, VacA activates the p38 and ERK-1/2 MAP kinases, thereby contributing to the induction of immune responses by these cells[43]. In fact, the vacA gene product has been shown to cause in mice, some of the tissue damage observed in H pylori-infected patients[44].
Another major virulence factor of H pylori is urease, which is expressed by all strains. Urease is composed of two subunits, α, which is approximately 24 kDa, and β, which is approximately 68 kDa. H pylori produces a large amount of urease, representing 5% to 10% of the total protein content of the bacteria. This enzyme is essential for the survival and pathogenesis of the bacteria. Perhaps the most important role urease plays is to hydrolyze urea into CO2 and NH3, which aids in buffering the bacteria from the acidic conditions it may encounter in the stomach. Urease is crucial for H pylori colonization, as shown by studies where urease negative strains were not able to colonize in multiple animal studies[45,46]. The inability of urease negative strains to colonize was initially assumed to be due to their inability to buffer their niche. However, similar studies under hypoacidic conditions also led to the same results, where urease negative mutants of H pylori could not colonize in an animal model. These observations suggested a role for urease beyond its enzymatic function. Although much urease is located intracellularly, there is some present on the bacterial surface[47,13]. H pylori surface-associated urease can act as an adhesin for the bacteria, which induces the production of inflammatory cytokines from both gastric epithelial cells and macrophages[48,49], along with apoptosis of some cells[11]. While the mechanism of action associated with these responses elevated by urease is not entirely clear, the induction of apoptosis may result as a consequence of binding to class II MHC[50]. The urease B subunit can also bind to CD74 and induce IL-8 production by gastric epithelial cells[51]. Both of these responses are important in the overall pathogenesis seen during H pylori infection.
Another important disease-associated virulence factor of H pylori is the outer inflammatory protein A (OipA). OipA is part of a family of 32 outer membrane proteins characterized as part of the H pylori genome. This protein has been suggested to induce pro-inflammatory responses from gastric epithelial cell lines. In one study with H pylori clinical isolates, those isolates expressing OipA, but not the cag pathogenicity island proteins were able to induce IL-8 production from gastric epithelial cell lines at 3 times the level of strains that did not express either[52]. Isolates from Japan all expressed OipA, while isolates from the U.S. did not, and thus it is thought that the presence of OipA may make Japanese strains more virulent. When the signaling induced by cagPAI proteins was compared to OipA, OipA was found to induce phosphorylation of Stat1, while the cagPAI proteins induced NF-κB activation[52]. Both of these signaling pathways contribute to induction of IL-8 production, but act in conjunction with one another to fully activate the IL-8 promoter.
Some other virulence factors expressed by H pylori include neutrophil-activating protein (NAP) and heat shock protein 60 (Hsp60)[53,54]. NAP has been shown to be a chemoattractant for both monocytes and neutrophils during H pylori infection[55]. Hsp60 has been shown to induce proinflammatory cytokines by macrophages and gastric epithelial cells[56,57], which appears to be mediated by Toll-like receptors (TLRs). Lipopolysaccharide expressed by H pylori is a very weak immunogen compared to that of other gram negative bacteria, but it has been shown to induce some proinflammatory cytokines[58]. Although the interactions that initiate epithelial cell signals following bacterial adherence are critical in pathogenesis, they are not well understood, nor are the responses of the gastric epithelium that contribute to chronicity of the infection.
In a VacA paralogue mechanism H pylori can secrete collagenase, which can degrade collagen present in the extracellular matrix to supply the bacteria with essential amino acids. During chronic infections, collagenase can exacerbate ulcer development and deter the process of ulcer healing[59].
Perhaps the most important response for the pathogenesis associated with infection is inflammation. Both antigen specific and non-specific responses contribute to inflammation during infection. These responses contribute to fighting infection, but are also responsible for mucosal damage. Adhesion of H pylori to the host epithelium, or bacterial factors such as urease or the cagPAI proteins, induce signaling that upregulates proinflammatory cytokines and chemokines such as IL-8 and GRO-α. In a recent study, we showed that CagA is important in the induction of macrophage migration inhibitory factor (MIF) production by the gastric epithelium[60]. This cytokine has significant importance in the innate and adaptive host responses. Although the cagPAI proteins are the most recognized factor inducing inflammatory responses, there are several other interactions known to upregulate these responses. We have recently discovered that through H pylori attachment to CD74, or crosslinking CD74 on gastric epithelial cells, NF-κB activation occurs leading to IL-8 production[15]. Blocking this interaction with monoclonal antibodies resulted in a substantial decrease in the amount of IL-8 produced in response to H pylori. Other bacterial factors that induce inflammatory responses are HSP60, which was shown to induce IL-8 through Toll-like receptor pathways[57], and urease, which induced responses from both gastric epithelial cells and peripheral blood mononuclear cells[49]. The cytokine responses, in turn, recruit other immune cells to the site of infection such as IL-8, which is one of the initial chemokines that recruits neutrophils to the site of infection. Neutrophils are then activated by H pylori or its soluble products, and proceed to release reactive oxygen species (ROS) and more IL-8[61], which lead to tissue damage associated with infection.
Another response of GECs to infection is enhanced proliferation. In order to balance the increased growth of epithelial cells, the host must compensate by increasing epithelial cell turnover. One mechanism to account for epithelial cell turnover is increased cell death. Apoptosis provides a highly regulated mechanism for cell loss in both healthy and inflamed tissue. In the digestive tract, apoptosis has been described as being important in the control of normal epithelial cell turnover while it is increased in the gastric epithelium during infection with H pylori[62,63]. The rate of apoptosis induction may be regulated by exogenous cytokines and growth factors. For example, we have shown that IFN-γ can directly augment the ability of H pylori to induce apoptosis of GECs[50]. An indirect effect of IFN-γ is its ability to induce an increased expression of putative receptors (i.e., class II MHC and CD74) for H pylori. Our studies have shown that H pylori induces the expression of class II MHC and CD74[50,64], and another study showed that H pylori induces yet another receptor, sialyl-Lex, on GEC[65].
Other cytokines and chemokines induced by H pylori may also play a role in inducing GEC proliferation. MIF, which is produced by GEC in response to CagA injection[60], and may be produced by macrophages and T cells during infection[66] can induce GEC proliferation. MIF affects proliferation by inactivating p53 tumor suppressor gene and inducing proliferative signaling such as ERK1/2 activation. Additionally, IL-8 has recently been shown to promote GEC proliferation by accelerating the processing of EGFR ligands, which bind and induce transactivation of the receptor[67]. In one study administering nonsteroidal anti-inflammatory drugs was capable of decreasing GEC proliferation in the mouse model[68], further suggesting a role for pro-inflammatory cytokines in cell turnover during H pylori infection.
The prevalence of IL-8 produced by the gastric epithelium at the site of infection results in the infiltration of neutrophils. H pylori soluble factors activate neutrophils[69], which go on to produce reactive oxygen species (ROS). Gastric epithelial cells have also been shown to produce ROS in response to H pylori[70]. Interestingly, H pylori appear to be resistant to the antimicrobial action of ROS. However, ROS may also induce DNA damage in the epithelium, which leads to apoptosis. Since ROS production appears to be dependant on bacterial load[71], it also may be correlated to the amount of damage to the epithelium. When there is a lower bacterial load, the balance between oxidants and antioxidants in the gastric mucosa is disrupted at levels not high enough to induce apoptosis. The risk of DNA damage from ROS is high, and thereby may lead to pro-carcinogenic events.
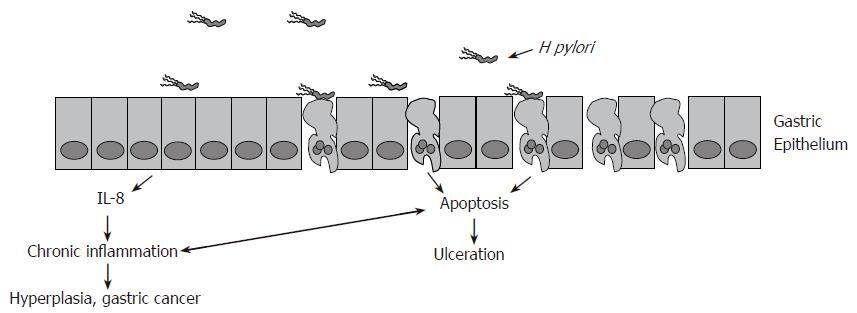
The interactions of H pylori with the host are a complex series of events that induce pathogenesis while allowing the bacterium to persist. Only about 20% of the bacteria are bound to the epithelium via multiple adhesions at any given time[72]. Attachment to the epithelium, along with multiple virulence factors, induce proinflammatory immune responses. These responses can affect host cell viability and lead to one of two mutually exclusive events. Either excess gastric acid is produced leading to ulceration, or chronic inflammation induces atrophy of the stomach wall and malignant outgrowths (Figure 3). The unique and persistent interactions of H pylori with the host, along with 50% worldwide infection rates, make it a significant pathogen that induces considerable disease.
While a significant volume of knowledge has been acquired during the last decade to help us understand how H pylori causes disease, there is still no available vaccine that is effective against this pathogen. H pylori’s ability to maintain its long-term residence in a broad segment of humankind is in large part due to the subversion of common structures on the host cells for its interactions. As with most infectious agents, adhesion to host cells is a crucial step in colonization. Attachment is facilitated by various structures or adhesins on the bacteria which include BabA, SabA, HopZ, AlpA/B, and urease. These adhesins bind to carbohydrate moieties on blood group antigens, as is the case of BabA and SabA binding to Lewis antigen, or to proteins of central importance to the host response, such as urease binding to class II MHC and CD74. These interactions are important to characterize in detail as they may offer insights into novel prophylactic or therapeutic agents directed at H pylori-associated diseases.
Following colonization, H pylori employs virulence factors, such as the cagPAI type IV secretion system and a vacuolating cytotoxin to exert damage on the host epithelium and to alter the host immune response. The ability of VacA to disrupt endosomal traffic and thus alter antigen presentation, together with its ability to arrest T cell cycle progression, makes VacA an important virulence factor that could contribute to the establishment of chronic infection. As we understand the mechanisms that contribute to the long term residence of H pylori in the human stomach, we will be better able to prevent the chronicity that underlies the development of the serious diseases associated with this infection.
S- Editor Liu Y L- Editor Misra SP E- Editor Bi L
| 1. | Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 230] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177-240. [PubMed] [Cited in This Article: ] |
| 3. | Cancer Database; Available from: http: //www-dep iarc fr/2005. . [Cited in This Article: ] |
| 4. | Nakamura S, Yao T, Aoyagi K, Iida M, Fujishima M, Tsuneyoshi M. Helicobacter pylori and primary gastric lymphoma. A histopathologic and immunohistochemical analysis of 237 patients. Cancer. 1997;79:3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 5. | Parsonnet J, Isaacson PG. Bacterial infection and MALT lymphoma. N Engl J Med. 2004;350:213-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Hayashi S, Sugiyama T, Asaka M, Yokota K, Oguma K, Hirai Y. Modification of Helicobacter pylori adhesion to human gastric epithelial cells by antiadhesion agents. Dig Dis Sci. 1998;43:56S-60S. [PubMed] [Cited in This Article: ] |
| 7. | Hennig EE, Mernaugh R, Edl J, Cao P, Cover TL. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect Immun. 2004;72:3429-3435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Unemo M, Aspholm-Hurtig M, Ilver D, Bergström J, Borén T, Danielsson D, Teneberg S. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J Biol Chem. 2005;280:15390-15397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Odenbreit S, Faller G, Haas R. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int J Med Microbiol. 2002;292:247-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Peck B, Ortkamp M, Diehl KD, Hundt E, Knapp B. Conservation, localization and expression of HopZ, a protein involved in adhesion of Helicobacter pylori. Nucleic Acids Res. 1999;27:3325-3333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol. 2000;165:1918-1924. [PubMed] [Cited in This Article: ] |
| 12. | Bode G, Malfertheiner P, Lehnhardt G, Nilius M, Ditschuneit H. Ultrastructural localization of urease of Helicobacter pylori. Med Microbiol Immunol. 1993;182:233-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Rokita E, Makristathis A, Hirschl AM, Rotter ML. Purification of surface-associated urease from Helicobacter pylori. J Chromatogr B Biomed Sci Appl. 2000;737:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Barrera CA, Beswick EJ, Sierra JC, Bland D, Espejo R, Mifflin R, Adegboyega P, Crowe SE, Ernst PB, Reyes VE. Polarized expression of CD74 by gastric epithelial cells. J Histochem Cytochem. 2005;53:1481-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Beswick EJ, Bland DA, Suarez G, Barrera CA, Fan X, Reyes VE. Helicobacter pylori binds to CD74 on gastric epithelial cells and stimulates interleukin-8 production. Infect Immun. 2005;73:2736-2743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | O'Brien DP, Israel DA, Krishna U, Romero-Gallo J, Nedrud J, Medof ME, Lin F, Redline R, Lublin DM, Nowicki BJ. The role of decay-accelerating factor as a receptor for Helicobacter pylori and a mediator of gastric inflammation. J Biol Chem. 2006;281:13317-13323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Leunk RD. Production of a cytotoxin by Helicobacter pylori. Rev Infect Dis. 1991;13 Suppl 8:S686-S689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Tee W, Lambert JR, Dwyer B. Cytotoxin production by Helicobacter pylori from patients with upper gastrointestinal tract diseases. J Clin Microbiol. 1995;33:1203-1205. [PubMed] [Cited in This Article: ] |
| 19. | Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22:515-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Figueiredo C, Machado JC, Yamaoka Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2005;10 Suppl 1:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Higashi H, Nakaya A, Tsutsumi R, Yokoyama K, Fujii Y, Ishikawa S, Higuchi M, Takahashi A, Kurashima Y, Teishikata Y. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J Biol Chem. 2004;279:17205-17216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 22. | Churin Y, Al-Ghoul L, Kepp O, Meyer TF, Birchmeier W, Naumann M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol Cell. 2002;10:745-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300-9305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 394] [Cited by in F6Publishing: 396] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 25. | Kim SY, Lee YC, Kim HK, Blaser MJ. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol. 2006;8:97-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 567] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 27. | Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 906] [Cited by in F6Publishing: 897] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 28. | Churin Y, Kardalinou E, Meyer TF, Naumann M. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol Microbiol. 2001;40:815-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Guillemin K, Salama NR, Tompkins LS, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc Natl Acad Sci USA. 2002;99:15136-15141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 173] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Keates S, Sougioultzis S, Keates AC, Zhao D, Peek RM Jr, Shaw LM, Kelly CP. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J Biol Chem. 2001;276:48127-48134. [PubMed] [Cited in This Article: ] |
| 31. | Umehara S, Higashi H, Ohnishi N, Asaka M, Hatakeyama M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337-8342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Kuo CH, Wang WC. Binding and internalization of Helicobacter pylori VacA via cellular lipid rafts in epithelial cells. Biochem Biophys Res Commun. 2003;303:640-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Schraw W, Li Y, McClain MS, van der Goot FG, Cover TL. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J Biol Chem. 2002;277:34642-34650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Szabò I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517-5527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 380] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 37. | Cover TL, Krishna US, Israel DA, Peek RM Jr. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951-957. [PubMed] [Cited in This Article: ] |
| 38. | Kimura M, Goto S, Wada A, Yahiro K, Niidome T, Hatakeyama T, Aoyagi H, Hirayama T, Kondo T. Vacuolating cytotoxin purified from Helicobacter pylori causes mitochondrial damage in human gastric cells. Microb Pathog. 1999;26:45-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 238] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Boncristiano M, Paccani SR, Barone S, Ulivieri C, Patrussi L, Ilver D, Amedei A, D'Elios MM, Telford JL, Baldari CT. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J Exp Med. 2003;198:1887-1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci USA. 2004;101:7727-7732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 395] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 43. | Nakayama M, Kimura M, Wada A, Yahiro K, Ogushi K, Niidome T, Fujikawa A, Shirasaka D, Aoyama N, Kurazono H. Helicobacter pylori VacA activates the p38/activating transcription factor 2-mediated signal pathway in AZ-521 cells. J Biol Chem. 2004;279:7024-7028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Telford JL, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce MF, Censini S, Covacci A, Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653-1658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 431] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 45. | Karita M, Tsuda M, Nakazawa T. Essential role of urease in vitro and in vivo Helicobacter pylori colonization study using a wild-type and isogenic urease mutant strain. J Clin Gastroenterol. 1995;21 Suppl 1:S160-S163. [PubMed] [Cited in This Article: ] |
| 46. | Tsuda M, Karita M, Morshed MG, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586-3589. [PubMed] [Cited in This Article: ] |
| 47. | Phadnis SH, Parlow MH, Levy M, Ilver D, Caulkins CM, Connors JB, Dunn BE. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905-912. [PubMed] [Cited in This Article: ] |
| 48. | Harris PR, Ernst PB, Kawabata S, Kiyono H, Graham MF, Smith PD. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J Infect Dis. 1998;178:1516-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Takahashi T, Yujiri T, Shinohara K, Inoue Y, Sato Y, Fujii Y, Okubo M, Zaitsu Y, Ariyoshi K, Nakamura Y. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori-associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 50. | Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley WK, Ernst PB, Reyes VE. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Beswick EJ, Pinchuk IV, Minch K, Suarez G, Sierra JC, Yamaoka Y, Reyes VE. The Helicobacter pylori urease B subunit binds to CD74 on gastric epithelial cells and induces NF-kappaB activation and interleukin-8 production. Infect Immun. 2006;74:1148-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533-7538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 305] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 53. | Bai Y, Li LR, Wang JD, Chen Y, Jin JF, Zhang ZS, Zhou DY, Zhang YL. Expression of Helicobacter pylori Hsp60 protein and its immunogenicity. World J Gastroenterol. 2003;9:2711-2714. [PubMed] [Cited in This Article: ] |
| 54. | Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Relationship between expression of HSP60, urease activity, production of vacuolating toxin, and adherence activity of Helicobacter pylori. J Gastroenterol. 1998;33 Suppl 10:6-9. [PubMed] [Cited in This Article: ] |
| 55. | Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Gobert AP, Bambou JC, Werts C, Balloy V, Chignard M, Moran AP, Ferrero RL. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a toll-like receptor (TLR)-2-, TLR-4-, and myeloid differentiation factor 88-independent mechanism. J Biol Chem. 2004;279:245-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Takenaka R, Yokota K, Ayada K, Mizuno M, Zhao Y, Fujinami Y, Lin SN, Toyokawa T, Okada H, Shiratori Y. Helicobacter pylori heat-shock protein 60 induces inflammatory responses through the Toll-like receptor-triggered pathway in cultured human gastric epithelial cells. Microbiology. 2004;150:3913-3922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Bliss CM Jr, Golenbock DT, Keates S, Linevsky JK, Kelly CP. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect Immun. 1998;66:5357-5363. [PubMed] [Cited in This Article: ] |
| 59. | Kavermann H, Burns BP, Angermuller K, Odenbreit S, Fischer W, Melchers K, Haas R. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J Exp Med. 2003;197:813-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 60. | Beswick EJ, Pinchuk IV, Suarez G, Sierra JC, Reyes VE. Helicobacter pylori CagA-dependent macrophage migration inhibitory factor produced by gastric epithelial cells binds to CD74 and stimulates procarcinogenic events. J Immunol. 2006;176:6794-6801. [PubMed] [Cited in This Article: ] |
| 61. | Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Helicobacter pylori water soluble surface proteins prime human neutrophils for enhanced production of reactive oxygen species and stimulate chemokine production. J Clin Pathol. 2003;56:348-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 331] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 63. | Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238-3243. [PubMed] [Cited in This Article: ] |
| 64. | Beswick EJ, Das S, Pinchuk IV, Adegboyega P, Suarez G, Yamaoka Y, Reyes VE. Helicobacter pylori-induced IL-8 production by gastric epithelial cells up-regulates CD74 expression. J Immunol. 2005;175:171-176. [PubMed] [Cited in This Article: ] |
| 65. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 626] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 66. | He XX, Yang J, Zheng XL, Ding YW, Shen QY, Liu W, Zhao YH. The effect of Helicobacter pylori infection on expression of macrophage migration inhibitory factor by T cells and macrophages in gastric mucosa. Chin Med J (Engl). 2005;118:1201-1205. [PubMed] [Cited in This Article: ] |
| 67. | Joh T, Kataoka H, Tanida S, Watanabe K, Ohshima T, Sasaki M, Nakao H, Ohhara H, Higashiyama S, Itoh M. Helicobacter pylori-stimulated interleukin-8 (IL-8) promotes cell proliferation through transactivation of epidermal growth factor receptor (EGFR) by disintegrin and metalloproteinase (ADAM) activation. Dig Dis Sci. 2005;50:2081-2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Kim TI, Lee YC, Lee KH, Han JH, Chon CY, Moon YM, Kang JK, Park IS. Effects of nonsteroidal anti-inflammatory drugs on Helicobacter pylori-infected gastric mucosae of mice: apoptosis, cell proliferation, and inflammatory activity. Infect Immun. 2001;69:5056-5063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Suzuki H, Miura S, Imaeda H, Suzuki M, Han JY, Mori M, Fukumura D, Tsuchiya M, Ishii H. Enhanced levels of chemiluminescence and platelet activating factor in urease-positive gastric ulcers. Free Radic Biol Med. 1996;20:449-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res. 1996;24:439-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Davies GR, Banatvala N, Collins CE, Sheaff MT, Abdi Y, Clements L, Rampton DS. Relationship between infective load of Helicobacter pylori and reactive oxygen metabolite production in antral mucosa. Scand J Gastroenterol. 1994;29:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Hessey SJ, Spencer J, Wyatt JI, Sobala G, Rathbone BJ, Axon AT, Dixon MF. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990;31:134-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 202] [Article Influence: 5.9] [Reference Citation Analysis (0)] |