Published online Jun 28, 2006. doi: 10.3748/wjg.v12.i24.3938
Revised: February 10, 2006
Accepted: February 20, 2006
Published online: June 28, 2006
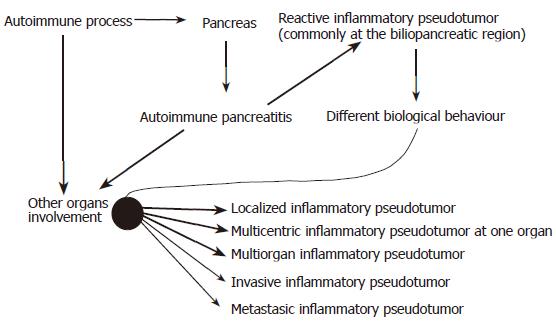
Inflammatory myofibroblastic tumors (IMTs) or inflammatory pseudotumors (IPs) have been extensively discussed in the literature. They are usually found in the lung and upper respiratory tract. However, reporting of cases involving the biliopancreatic region has increased over recent years. Immunohistochemical study of these lesions limited to the pancreatic head or distal bile duct seems to be compatible with those observed in a new entity called autoimmune pancreatitis, but usually intense fibrotic reaction (zonation) predominates producing a mass. When this condition is limited to the pancreatic head, the common bile duct might be involved by the inflammatory process and jaundice may occur often resembling adenocarcinoma of the pancreas. We have previously reported a case of IMT arising from the bile duct associated with autoimmune pancreatitis which is an extremely rare entity. Four years after Kaush-Whipple resection, radiological examination on routine follow-up revealed a tumor mass, suggesting local recurrence. Ultrasound-guided FNA confirmed our suspicious diagnosis. This present case, as others, suggests that persistent follow-up is necessary in order to prevent irreversible liver damage at this specific location.
- Citation: Fernández ELT, Luis HD, Malagón AM, González IA, Pallarés AC. Recurrence of inflammatory pseudotumor in the distal bile duct: Lessons learned from a single case and reported cases. World J Gastroenterol 2006; 12(24): 3938-3943
- URL: https://www.wjgnet.com/1007-9327/full/v12/i24/3938.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i24.3938
Histologically, inflammatory myofibroblastic tumor (IMT) is characterized by a proliferation of spindle cells admixed with variable amounts of a lymphoplasmacytic infiltrate. These changes in the pancreatic head produce a mass effect in the biliopancreatic region mimicking a malignant tumor. Thus, surgical resection has been often necessary in order to obtain a definitive diagnosis as well as to relieve symptoms[1]. IMT has been shown to possess potentiality for local infiltration and recurrence with persistent local growth at any organ. Hence, a strict follow-up after surgery is necessary because local recurrence may occur many years later. We report herein a case of local IMT recurrence four years after Kausch-Whipple resection with tumor-free margins in a 55-year-old woman. Primary tumor was located in the distal common bile duct accompanied with lymphoplasmacytic reaction in the pancreatic head. To our best of knowledge, this is the first case reporting a local reappearance of inflammatory pseudotumor in the distal bile duct associated with benign lymphoplasmacytic sclerosing pancreatitis that responded successfully to corticosteroid therapy. Further follow-up in the present case is necessary to elucidate if it behaves as a reactive progressive lesion or tumor.
A 55-year-old woman with a past history of IMT affecting the biliopancreatic region [myofibroblastic inflammatory tumor of the distal bile duct (OCD-O code C-24.O M-8825/1) associated with lymphoplasmacytic sclerosing pancreatitis, chronic cholecystitis and reactive lymphadenitis of the isolated nodes] was on routine follow-up after Kaush-Whipple resection.
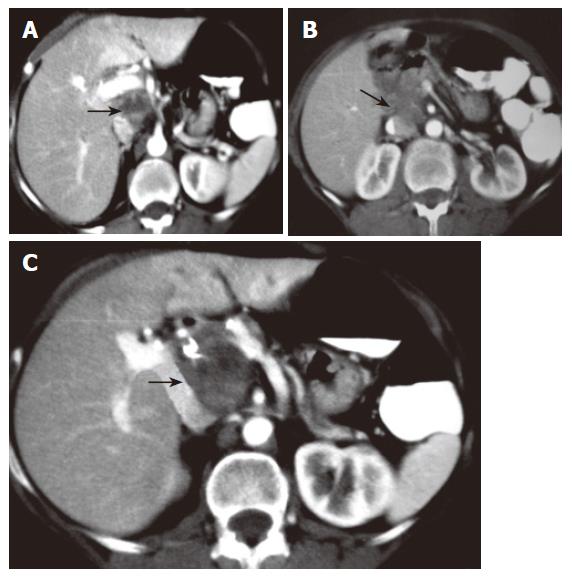
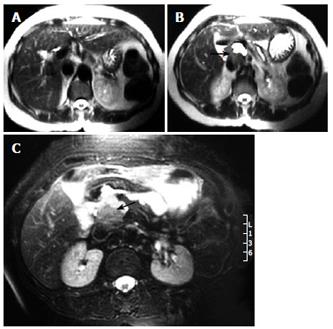
Four years later, routine surveillance CT scans detected a well defined mass in close relation to the portal vein without apparent infiltration of surrounded structures. At this time, weight loss, malaise or anemia were not present. The tumor mass was well circumscribed, measuring 5 cm × 5 cm, had solid pattern and did not enhance with contrast (Figure 1). An abdominal MRI further delineated that the mass was located between the portal vein and inferior vena cava with no signs of widespread infiltration of organs, thereby strongly suggesting the diagnosis of local recurrence (Figure 2). It did not reveal intrahepatic bile duct dilatation but did show chronic changes in pancreatic tissue (Figure 3). Ultrasound-guided fine-needle aspiration (FNA) was performed for cytological examination, which revealed prominent infiltrate of lymphocytes, plasma cells, and acute inflammatory cells without cytologic atypia. These findings were compatible with IMT. Therefore, local recurrence showing benign behaviour was suspected. Then, without evidence of systemic disease, corticosteroid therapy was started. A repeat ultrasound revealed complete remission of the tumor at the 3rd mo. The steroid dose is being tapered downward gradually, and we might expect complete remission 6 mo later on low-dose steroid maintenance treatment. On the 5th mo of therapy, the patient remains well and liver function tests were within normal limits.
Although IMTs generally behave as benign lesions that are usually cured by radical excision, some cases demonstrate an aggressive growth behaviour. In a study conducted by Coffin et al[2], a significant recurrence rate of 25% was demonstrated, likely related to factors precluding complete surgical resection of the pseudotumor. There is a tendency to believe that IMT occurring in the abdomen or retroperitoneum has a propensity for more aggressive behavior with multiple recurrences, invasion into adjacent structures and metastases as shown by DiFiore et al[3] and Meis et al[4]. However, other recent reports showed that aggressive behaviour, rapid infiltration and multiple recurrences of IMTs are not limited to abdominal location[5-7]. Nowadays, limited evidences exist on the outcomes of patients with a local recurrence of IMT from the biliopancreatic region.
A recent review[1] mentioned reported cases of IMT at the biliopancreatic region, describing locoregional recurrences of four cases at the biliopancreatic region[8,9] after primary resection of the tumor. Further study of these cases showed that just only one of the four cases represented a true local recurrence of pseudotumor arising from the bile duct. The other three cases were much different from ours because primary tumor mass was located in the pancreas or because the recurrence was found at a distant site (lung) or the recurrence occurred after a long time interval (17 years) from resection and at a different site from primary tumor. The case reported by Johnson et al[9] in a 29-year-old black woman recurred after Kaush-Whipple procedure 8 mo later at original site, although the tumor mass was primarily located in the pancreas tissue. Walsh et al[8] reported three cases of IMT affecting the biliopancreatic region with long-term follow-up. In one of them, recurrence was found at a distant site (lung). In other case, there was a long interval from resection to recurrence in the pancreatic tail, reason why is not considered a true recurrence. If these recurrences represent a second tumor, multicentricity or metastasis is unknown. In the third case, the tumor recurred at the original site (right main hepatic duct) 14 years later, extending into the liver and showed a benign behaviour. This latter case requires further attention as it is similar to ours but has some interesting differences.
It is widely believed today that IMT at the biliopancreatic region, specially distal bile duct, are usually associated with a new entity called autoimmune pancreatitis which is believed to be an initial activation process. There are clear evidences in literature that most of the reported cases of IMT from the distal bile duct showed association with sclerosing lymphoplasmacytic pancreatitis or autoimmune pancreatitis[1,10-13] and indicates that the fibroblastic cells in both lesions may be induced at the same site and are usually the same inflammatory and fibrosing process with different histological appearances. However, Walsh et al[8] did not mention whether the primary tumor at the common bile duct was associated with any form of pancreatitis. Pancreatic tissue was not studied, even they did not mention whether the distal margin was free of inflammatory process. Whether this case represents a form of cholangitis associated with lymphoplasmacytic sclerosing pancreatitis is unknown.
Thus, the case reported by Walsh et al[8] that did not show any association with autoimmune process is different from ours in which the distal bile duct and pancreatic head was involved by the same inflammatory process. Other important point is that final post-mortem study of the specimen from the case of Walsh et al[8] showed involvement of the liver which suggests and reinforces previous theories that there is possibly an idiopathic pancreatobiliary inflammatory disease complex involving various processes such as autoimmune pancreatitis, although this was not demonstrated.
Apart from this reported cases, our case represent a true local recurrence of pseudotumor of the distal bile duct associated with autoimmune process in the pancreas head. The present case confirms that IMTs have a tendency to recur with time even after radical excision of primary tumor with free margins. These findings support the theory that IMTs are considered as a systemic disease with different pattern of behaviour in which persistent autoimmune reaction process and multicentricity might be involved[13,14].
Our case seem to accord with the theories previously developed by Kawaguchi[15], Zen[13,16] and Klöppel[17] which associates autoimmune pancreatitis with variable involvement of the pancreas and the biliary tract. Fibrosis and zonation[2] may occur as a consequence of persitent inflammatory autoimmune process and biliary stenosis might developed in two different ways: due to external compression from fibrotic changes in the pancreatic head and secondary to zonation of the inflammatory process in the biliary tract which represents our case.
This report provides many important messages. First, local recurrence of IMT at biliopancreatic region has been rarely described. To our knowledge, no cases have been reported in the modern era, which usually associate pseudotumor formation in the distal bile duct with a new entity called autoimmune pancreatitis representing zonation of the same inflammatory process. Second, the biologic potential of inflammatory pseudotumors is highly variable (depending on its location and likely related to factors precluding complete surgical resection, such as adherence to vital structures and multifocality), but it generally has an innocuous course, with more local recurrences than distant metastasis as in our case.
Recurrence is common and the use of corticosteroids is permitted. In these cases, as shown by Voss et al[18] and our present case, local recurrences and systemic disease might not require surgery. However, surgery cannot be excluded and long-term follow-up is necessary in order to prevent irreversible liver damage in this specific location. Usually, acute lesions typically respond to high doses of corticosteroid[10], but chronic lesions such as recurrences, which tend to have more fibrosis, might not respond to medical therapy. Third, our case seems to reinforce the hypothesis that inflammatory myofibroblastic tumors from the bile duct might represent zonation by proximity of an autoimmune process affecting the pancreas. This might be explained by pattern of recurrence: our patient presented 4 years ago with indolent jaundice accompanied by biliary dilatation by inflammatory pseudotumor of the distal bile duct. She underwent Whipple procedure with tumor-free margins and negative isolated nodes. However, 4 years later, remaining asymptomatic, radiologic examinations revealed a tumor mass close to the primary lesion without bile duct compression. Moreover, MRI, liver function test and bilirrubin levels were normal. As Whipple procedure separates bile duct from pancreas tissue by interposed bowel loop, where did this recurrence come from What does the recurrence signify Perhaps this might be explained by new pseudotumor formation mediated by autoimmune process against remaining pancreatic tissue. Obviously, multicentricity or a new second tumor cannot be excluded.
Many questions still arise about the increasing appearance of inflammatory pseudotumors at different sites: What does really represent inflammatory myofibroblastic tumor High concentrations of serum Ig subtype, such as IgG4, in IMTs suggest that immunoglobulins may have a pathological role in the fibrosing process mediated by an autoimmune process. Broad pattern of presentation and its ample relation with other autoimmune diseases reinforce this theory[13,16,19-21].
Among other hypothesis, this theory is being increasingly recognized and reflects that inflammatory pseudotumors might represent an advanced stage of an autoimmune process in which intense fibrotic reaction (zonation) predominates[2,17]. What evidences exist in favour of the theory that IMTs at the biliopancreatic region are secondary to autoimmune process in the pancreas Possible evidences are the frequent occurrence of various autoimmune antibodies, such as antibodies against carboanhydrase II and nuclear antigens[22], the elevated IgG4 serum levels and IgG4 positive plasma cells[13,16,20,23], the oligoclonal pattern of T cell receptor gamma gene rearrangements[24], its association with reactive hyperplastic lymphadenopathy[1,8,25,26], and the responsiveness to steroid therapy of the reported cases[10,27-29]. More importantly, immunohistochemical results of these tumors are compatible with those of other autoimmune diseases. Is it a systemic disease when found at the biliopancreatic region whose course is determined by its biological activity Strong association between these tumors and other autoimmune processes suggest that IMTs are advanced reactive lesions secondary to an unidentified agent. High concentrations of serum IgG4 found in reported cases at various sites supports that IMTs represent a systemic condition. Hence, it may be localized, multicentric or systemic at the time of presentation and has a strong tendency to reccur. When found at the biliopancreatic region it may represent more than just a biliopancreatic disease.
Some evidences exist about its different biological behaviours that may represent a premalignant state[2,4,5,29-31]. What is the pattern of behaviour of inflammatory pseudotumors at the biliopancreatic region Limited evidence exists to respond this question, but usually behaves as a benign lesion as reported in the literature to date. Our case represents a local recurrence or multicentricity of IMT at the biliopancreatic region that responds well to conservative treatment. A series of four patients previously described by Walsh et al[8] and Voss et al[18] demonstrated that IMT from the biliopancreatic region showed a tendency to progressive involvement of multiple organ systems by the same histopathologic process at follow-up.
Is it possible to diagnose preoperatively an inflammatory myofibroblastic tumor presenting with jaundice at the biliopancreatic region and then avoid a complex surgical procedure A high index of suspicion is necessary to diagnose preoperatively this kind of tumor and exclude malignancy. Medical past history relating other autoinmmune process or previously diagnosed inflammatory pseudotumors at any site should be investigated. FNA with inmmunohistochemical study, and a good response to steroid therapy is necessary to avoid a surgical procedure. Distinguishing inflammatory pseudotumors from malignancy is extremely difficult to do on the basis of radiographic, ultrasound and MRI findings[1,33], specially at the biliopancreatic region as shown by Venkataraman et al[34]. Agrons et al[35] agreed with them but referring to extra-abdominal sites (lung). The heightened awareness should prompt more extensive non-invasive and minimally invasive radiologic, histopathologic, and inmmunohistochemical evaluations before directing patients toward complicated and potentially high-risk surgical procedures.
Further investigation will improve our understanding of pathophysiology and etiology of these lesions and such data will help us better recognize this entity preoperatively and differentiate it from carcinomas in order to avoid a surgical procedure. A well illustrated case was the one described by Sasahira et al[36].
How should we follow-up these patients Most organ systems may be involved by primary inflammatory pseudotumors; but also progressive multisystemic organ at follow-up has been described (Figure 4F)[18]. It also has been demonstrated that these chronic process may represent an indolent premalignant state[5,30,31]. Therefore, it is important to follow-up these patients to rapidly diagnose local or multisystemic recurrences in order to avoid complications specifically at the the biliopancreatic region where these compressing lesions might cause liver damage, cholangitis or pancreatitis. Follow-up must not be limited to the biliopancreatic region but also to other systemic organs. Increase rate in specific IgG4 might indicate reappearance of the autoimmune disease and become a useful marker to detect pseudotumor recurrence[37], but this issue remains unresolved and controversial[36].
The recurrence in our case is, in fact, represented by multicentricity of the primary tumor, a local recurrence or even systemic disease mediated by an autoimmune process. Overall, we must emphasize that such lesions respond well to corticosteroid therapy and high index of suspicion avoids a surgical procedure.
In conclusion, it seems to be only a question of time until inflammatory pseudotumor will reappear at any site once diagnosed. These lesions most likely will not appear until the etiology is more clearly defined and thus further studies should make a greater emphasis on etiologic factors rather than its presentation form, as virtually any organ may be involved.
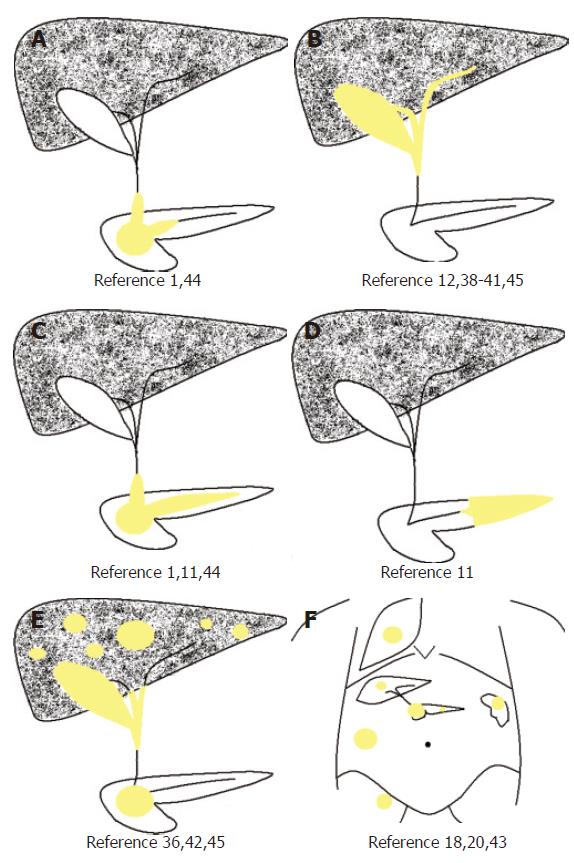
Steroid therapy should be the treatment of choice in recurrent cases as good responses have been found in the present and other cases. Follow-up is necessary to avoid complications from recurrences at the biliopancreatic region, especially to avoid liver damage as interval from silent cirrhosis to the development of complication may vary widely. We proposed a new dynamic classification for autoimmune pancreatic-related IMTs[17], with special attention at the biliopancreatic region (Figure 4)[1,11,12,18,20,36,38-44]. Among other theories[45-51], the most widely accepted theory today is that IMT is an auto-immune disorder (Figure 5). IgG4 can be useful as a serological marker of autoimmune pancreatitis and seems also to be useful for the follow-up of patients treated with steroids.
S- Editor Wang J L- Editor Mumar M E- Editor Bai SH
| 1. | Martín Malagón A, López-Tomassetti Fernández E, Arteaga González I, Carrillo Pallarés A, Díaz Luis H. Inflammatory myofibroblastic tumor of the distal bile duct associated with lymphoplasmacytic sclerosing pancreatitis. Case report and review of the literature. Pancreatology. 2006;6:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1100] [Cited by in F6Publishing: 980] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 3. | Difiore JW, Goldblum JR. Inflammatory myofibroblastic tumor of the small intestine. J Am Coll Surg. 2002;194:502-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146-1156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 298] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Donner LR, Trompler RA, White RR 4th. Progression of inflammatory myofibroblastic tumor (inflammatory pseudotumor) of soft tissue into sarcoma after several recurrences. Hum Pathol. 1996;27:1095-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Corneli G, Alifano M, Forti Parri S, Lacava N, Boaron M. Invasive inflammatory pseudo-tumor involving the lung and the mediastinum. Thorac Cardiovasc Surg. 2001;49:124-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Gale N, Zidar N, Podboj J, Volavsek M, Luzar B. Inflammatory myofibroblastic tumour of paranasal sinuses with fatal outcome: reactive lesion or tumour. J Clin Pathol. 2003;56:715-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Walsh SV, Evangelista F, Khettry U. Inflammatory myofibroblastic tumor of the pancreaticobiliary region: morphologic and immunocytochemical study of three cases. Am J Surg Pathol. 1998;22:412-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Johnson RL, Page DL, Dean RH. Pseudotumor of the pancreas. South Med J. 1983;76:647-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Hirano K, Shiratori Y, Komatsu Y, Yamamoto N, Sasahira N, Toda N, Isayama H, Tada M, Tsujino T, Nakata R. Involvement of the biliary system in autoimmune pancreatitis: a follow-up study. Clin Gastroenterol Hepatol. 2003;1:453-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Pungpapong S, Geiger XJ, Raimondo M. Inflammatory myofibroblastic tumor presenting as a pancreatic mass: a case report and review of the literature. JOP. 2004;5:360-367. [PubMed] [Cited in This Article: ] |
| 12. | Gumbs AA, Kim J, Kiehna E, Brink JA, Salem RR. Autoimmune pancreatitis presenting as simultaneous masses in the pancreatic head and gallbladder. JOP. 2005;6:455-459. [PubMed] [Cited in This Article: ] |
| 13. | Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis. Am J Surg Pathol. 2004;28:1193-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 14. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1011] [Cited by in F6Publishing: 924] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 15. | Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 490] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, Nakanuma Y. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005;29:275-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Klöppel G, Lüttges J, Sipos B, Capelli P, Zamboni G. Autoimmune pancreatitis: pathological findings. JOP. 2005;6:97-101. [PubMed] [Cited in This Article: ] |
| 18. | Voss SD, Kruskal JB, Kane RA. Chronic inflammatory pseudotumor arising in the hepatobiliary-pancreatic system: progressive multisystemic organ involvement in four patients. AJR Am J Roentgenol. 1999;173:1049-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Zen Y, Kitagawa S, Minato H, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Fujimura M, Nakanuma Y. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol. 2005;36:710-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, Egawa N, Nakajima H. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Hayashi Y, Funata N. Gastrointestinal findings in patients with autoimmune pancreatitis. Endoscopy. 2005;37:1127-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, Matsushima Y, Katamura K, Ohmori K, Chiba T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 428] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2026] [Cited by in F6Publishing: 1802] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 24. | Esposito I, Bergmann F, Penzel R, di Mola FF, Shrikhande S, Büchler MW, Friess H, Otto HF. Oligoclonal T-cell populations in an inflammatory pseudotumor of the pancreas possibly related to autoimmune pancreatitis: an immunohistochemical and molecular analysis. Virchows Arch. 2004;444:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Kojima M, Nakamura S, Oyama T, Motoori T, Itoh H, Yoshida K, Suchi T, Masawa N. Autoimmune disease-associated lymphadenopathy with histological appearance of T-zone dysplasia with hyperplastic follicles. A clinicopathological analysis of nine cases. Pathol Res Pract. 2001;197:237-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Manganelli P, Fietta P, Martella EM, Quaini F. Clinical and histological coexistence of inflammatory pseudotumour of the lymph nodes and rheumatoid arthritis. Clin Rheumatol. 2003;22:467-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Chutaputti A, Burrell MI, Boyer JL. Pseudotumor of the pancreas associated with retroperitoneal fibrosis: a dramatic response to corticosteroid therapy. Am J Gastroenterol. 1995;90:1155-1158. [PubMed] [Cited in This Article: ] |
| 28. | Erkelens GW, Vleggaar FP, Lesterhuis W, van Buuren HR, van der Werf SD. Sclerosing pancreato-cholangitis responsive to steroid therapy. Lancet. 1999;354:43-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Okazaki K. Autoimmune-related Pancreatitis. Curr Treat Options Gastroenterol. 2001;4:369-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Biselli R, Ferlini C, Fattorossi A, Boldrini R, Bosman C. Inflammatory myofibroblastic tumor (inflammatory pseudotumor): DNA flow cytometric analysis of nine pediatric cases. Cancer. 1996;77:778-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 31. | Snyder CS, Dell'Aquila M, Haghighi P, Baergen RN, Suh YK, Yi ES. Clonal changes in inflammatory pseudotumor of the lung: a case report. Cancer. 1995;76:1545-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 32. | Sciot R, Dal Cin P, Fletcher CD, Hernandez JM, Garcia JL, Samson I, Ramos L, Brys P, Van Damme B, Van den Berghe H. Inflammatory myofibroblastic tumor of bone: report of two cases with evidence of clonal chromosomal changes. Am J Surg Pathol. 1997;21:1166-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Inaba K, Suzuki S, Yokoi Y, Ota S, Nakamura T, Konno H, Baba S, Takehara Y, Nakamura S. Hepatic inflammatory pseudotumor mimicking intrahepatic cholangiocarcinoma: report of a case. Surg Today. 2003;33:714-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Venkataraman S, Semelka RC, Braga L, Danet IM, Woosley JT. Inflammatory myofibroblastic tumor of the hepatobiliary system: report of MR imaging appearance in four patients. Radiology. 2003;227:758-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Agrons GA, Rosado-de-Christenson ML, Kirejczyk WM, Conran RM, Stocker JT. Pulmonary inflammatory pseudotumor: radiologic features. Radiology. 1998;206:511-518. [PubMed] [Cited in This Article: ] |
| 36. | Sasahira N, Kawabe T, Nakamura A, Shimura K, Shimura H, Itobayashi E, Asada M, Shiratori Y, Omata M. Inflammatory pseudotumor of the liver and peripheral eosinophilia in autoimmune pancreatitis. World J Gastroenterol. 2005;11:922-925. [PubMed] [Cited in This Article: ] |
| 37. | Hirano K, Komatsu Y, Yamamoto N, Nakai Y, Sasahira N, Toda N, Isayama H, Tada M, Kawabe T, Omata M. Pancreatic mass lesions associated with raised concentration of IgG4. Am J Gastroenterol. 2004;99:2038-2040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Ikeda H, Oka T, Imafuku I, Yamada S, Yamada H, Fujiwara K, Hirata M, Idezuki Y, Oka H. A case of inflammatory pseudotumor of the gallbladder and bile duct. Am J Gastroenterol. 1990;85:203-206. [PubMed] [Cited in This Article: ] |
| 39. | Corsi A, Bosman C. Chronic cholecystitis with features of diffuse inflammatory pseudotumour: a clinico-pathological case study and review of the literature. Ital J Gastroenterol. 1995;27:252-255. [PubMed] [Cited in This Article: ] |
| 40. | Nonomura A, Minato H, Shimizu K, Kadoya M, Matsui O. Hepatic hilar inflammatory pseudotumor mimicking cholangiocarcinoma with cholangitis and phlebitis--a variant of primary sclerosing cholangitis. Pathol Res Pract. 1997;193:519-525; discussion 526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Behranwala KA, Straker P, Wan A, Fisher C, Thompson JN. Inflammatory myofibroblastic tumour of the gallbladder. World J Surg Oncol. 2005;3:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Kanno A, Satoh K, Kimura K, Masamune A, Asakura T, Unno M, Matsuno S, Moriya T, Shimosegawa T. Autoimmune pancreatitis with hepatic inflammatory pseudotumor. Pancreas. 2005;31:420-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Aoki S, Nakazawa T, Ohara H, Sano H, Nakao H, Joh T, Murase T, Eimoto T, Itoh M. Immunohistochemical study of autoimmune pancreatitis using anti-IgG4 antibody and patients' sera. Histopathology. 2005;47:147-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Zanger P, Kronsbein U, Merkle P, Bosse A. [Inflammatory myofibroblastic tumor of the pancreas with regional lymph node involvement]. Pathologe. 2002;23:161-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Eckstein RP, Hollings RM, Martin PA, Katelaris CH. Pancreatic pseudotumor arising in association with Sjögren's syndrome. Pathology. 1995;27:284-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Arber DA, Kamel OW, van de Rijn M, Davis RE, Medeiros LJ, Jaffe ES, Weiss LM. Frequent presence of the Epstein-Barr virus in inflammatory pseudotumor. Hum Pathol. 1995;26:1093-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 253] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 47. | Selves J, Meggetto F, Brousset P, Voigt JJ, Pradère B, Grasset D, Icart J, Mariamé B, Knecht H, Delsol G. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein-Barr virus. Am J Surg Pathol. 1996;20:747-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Janigan DT, Marrie TJ. An inflammatory pseudotumor of the lung in Q fever pneumonia. N Engl J Med. 1983;308:86-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Thomas RM, Jaffe ES, Zarate-Osorno A, Medeiros LJ. Inflammatory pseudotumor of the spleen. A clinicopathologic and immunophenotypic study of eight cases. Arch Pathol Lab Med. 1993;117:921-926. [PubMed] [Cited in This Article: ] |
| 50. | Jaïs P, Berger JF, Vissuzaine C, Paramelle O, Clays-Schouman E, Potet F, Mignon M. Regression of inflammatory pseudotumor of the liver under conservative therapy. Dig Dis Sci. 1995;40:752-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Torzilli G, Inoue K, Midorikawa Y, Hui AM, Takayama T, Makuuchi M. Inflammatory pseudotumors of the liver: prevalence and clinical impact in surgical patients. Hepatogastroenterology. 2001;48:1118-1123. [PubMed] [Cited in This Article: ] |