Published online May 14, 2006. doi: 10.3748/wjg.v12.i18.2890
Revised: December 28, 2005
Accepted: January 14, 2006
Published online: May 14, 2006
AIM: To study the antitumor effect of Chinese compound Jinlongshe (JLS) granules on sarcoma 180 and MKN-45 human gastric cancer cell lines in vivo and its mechanism.
METHODS: After establishment of S180 sarcoma (S180) and MKN-45 gastric cancer model of nude mice, the tumor-bearing mice were divided into 5 groups at random. Three experimental groups were respectively given the aqueous extract of JLS granules at doses of 120 g, 60 g and 20 g /(kg per 6/wk, i.g) for 3 wk in S180 and 6 wk in nude mice model. Positive control was given cyclophosphamide (Cy) at a dose of 50 mg/(kg per 3 /wk, i.g) for 3 wk in S180 models and 5- Fluorouracil (5-FU) 20 mg/(kg per 3 /wk, i.g) for 3 wk in nude mice model. Negative control was given normal saline (NS) at a dose of 0.18 g/(kg per 6/wk, i.g) respectively. After 3 wk in mice bearing S180 tumor and 6 wk in nude mice model, the experimental animals were sacrificed and the masses of tumor were weighed, and the rates of tumor inhibition of each treated group were calculated respectively. To determine the antitumor mechanisms, the morphological changes, cell cycle and apoptosis were observed in MKN-45 nude mice model. Annexin V-FITC/PI double staining FCM assay was used to further determine the live cells, apoptotic cells, necrotic cells and debris.
RESULTS: The inhibitory rates of JLS granules at the doses of 20 g/kg, 60 g/kg and 120 g/kg were 50.31%, 55.94% and 68.13% (P < 0.01) in nude mice models and 40.90%, 50.32% and 58.46% (P < 0.01) in S180 model. The inhibitory rate of Cy was 85.22% in S180 models and the inhibitory rate of 5-FU was 53.43% in nude mice model (P < 0.01). Nuclear chromatin and margination were observed under a transmission electron microscope (TEM). The G0/G1 phase was arrested, typical apoptotic peak appeared, the apoptotic rate was 22.81%-38.54% in three JLS granule-treated groups. Annexin V-FITC/PI double staining FCM assay showed that the apoptotic cells were 4.36%, 3.08%and 7.08% in three dosages, most cells were localized in the low right quadrant.
CONCLUSION: Jinlongshe granules possess anti-tumor effects on experimental tumor models in vivo, and apoptosis induction is one of its anti-tumor mechanisms.
-
Citation: Yu ZH, Wei PK, Xu L, Qin ZF, Shi J. Anticancer effect of Jinlongshe granules on
in situ -transplanted human MKN-45 gastric cancer in nude mice and xenografted sarcoma 180 in Kunming mice and its mechanism. World J Gastroenterol 2006; 12(18): 2890-2894 - URL: https://www.wjgnet.com/1007-9327/full/v12/i18/2890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i18.2890
Gastric cancer is one of the most leading causes of cancer-related deaths worldwide[1]. Despite its decreasing trend in the Western world during the recent two decades, gastric cancer still accounts for about 23.2% of all cancer deaths in China[2-4]. Although surgery, chemotherapy, and irradiation are the mainstream therapeutic methods for gastric cancer, undesirable chemotherapy reaction and poor prognosis in the advanced gastric cancer patients are still the major problem for gastric cancer treatment[5]. Consequently, combination of multi-therapeutic methods may effectively improve gastric cancer patient’s symptoms and prolong their survival time[6-7].
Jinlongshe (JLS) granules are an oral Chinese medicine compound, consisting of Chinese herbs (Rhizome Arisaemat, Rhizoma Pinelliae, corium stomachium galli, Radix Glycyrrhizae preparata, etc.), developed by our department based on TCM theories of “phlegm” as the key factor in preventing and treating gastric cancer[8]. However, the exact antitumor mechanism of JLS granules is unclear. This study was to observe the anti-tumor effect of JLS granules and to determine its mechanism using sarcoma 180 and human gastric cancer cell line MKN-45 in vivo.
BALB/C nude mice (19-22 g) and Kunming mice (20-22 g) were provided by Experimental Animal Center of Chinese Academy of Sciences, Shanghai, China. All experimental animals were housed in specific-pathogen free (SPF) condition for 1 wk to adapt the surroundings before initiation of the experiment and had free access to food and water throughout the study.
S180 cell line and MKN-45 human gastric denocarcinoma cell line were provided by Institute of Pharmaceutical Research, Shanghai, Chinese Academy of Sciences.
Crude JLS granules were purchased from Leiyunshang Company, Shanghai, China. An aqueous extract of JLS granules at a concentration of 6 g/mL of raw materials was provided by Shanghai Changzheng Hospital. The aqueous extract was diluted with purified water before use. Cyclophosphamide injection (Cy, Lot No. 040909) was produced in Hualian Pharmaceutical Co., Ltd, Shanghai, China. OB Biogel (Lot No.040514) was produced in Baiyun Medical Biogel Co., Ltd, Guangzhou, China. Ketamine hydrochloride injection (Lot No.02072332) was produced in Hengrui Pharmaceutical Co., Ltd, Jiangsu, China.
The procedure of in situ-transplanted gastric cancer nude mouse model was referred to Xu and Chen Ling et al[9-10]. In brief, before transplantation, nude mice bearing gastric denocarcinoma MKN-45 cell lines were killed. Then the tumor was dissected and put into aseptical saline. Pieces of intact, fish meat-like tumor tissue of 1 mm × 1 mm × 1 mm in diameter from margin were prepared after removal of tumor capsule. Mice anesthetized with ketamine (50 mg/kg, ip) were sterilized with caseoiodine and a transverse incision was made on the left upper quadrant. After exposure of the greater curvature of stomach, serosa and muscular layer were incised with needle. Then tumor tissues were transplanted under serosa of stomach and one drop of OB biogel was applied to the tumor tissue. After 40 s, stomach was brought into abdominal cavity and abdominal wall was finally closed. The whole operation procedure was completed following the doctrine of aseptic manipulation.
This model was by subcutaneous injection as previously described[11]. Briefly, the S180 mouse sarcoma cells with ascites were harvested, diluted with sterilized saline at a ratio of 1:8 (cell concentration was adjusted to 1.0 × 106/mL), and inoculated subcutaneously into the right armpit region of Kunming mice.
Forty-eight hours after establishment of the MKN-45 gastric cancer model and 24 h after establishment of S180 model, the transplanted mice were divided into 5 groups according to the method described in “National Requirements for in vivo Screening of Anti-neoplastic Drugs”[12]. The dose of JLS granules was decided according to the clinical dose used in humans. The mice bearing S180 were killed by dislocation of cervical vertebra 3 wk after treatment. The nude mice bearing in situ-transplanted MKN-45 gastric cancer cell line were sacrificed 6 wk after treatment. The tumor weights were measured and the inhibitory rates were calculated according to the following equation: rate of inhibition (%) = (mean tumor wt of negative control -mean wt of treated group)/mean tumor wt of negative control ×100.
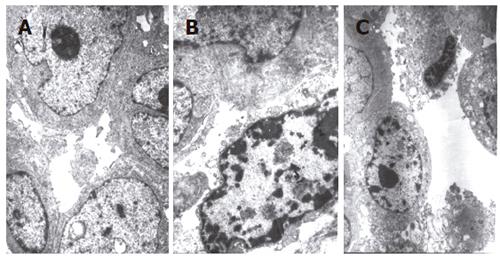
We selected NS group, 5-FU group and 60 g/kg JLS granules group for morphologic assessment of apoptosis in MKN-45 nude mice. Tumor tissue was cut into small pieces, homogenized into 1 mm × 2 mm, prefixed with 4% paraformaldehyde for 4 h and washed with PBS (0.1 mmol/L), post-fixed with 1% osmic acid, dehydrated in graded ethanol-acetonum, embedded in an Epon 812 mixture, and cut into sections on a ultramicrotome. After stained with uranyl acetate and lead citrate, the sections were examined under a Hitachi-800 transmission electron microscope.
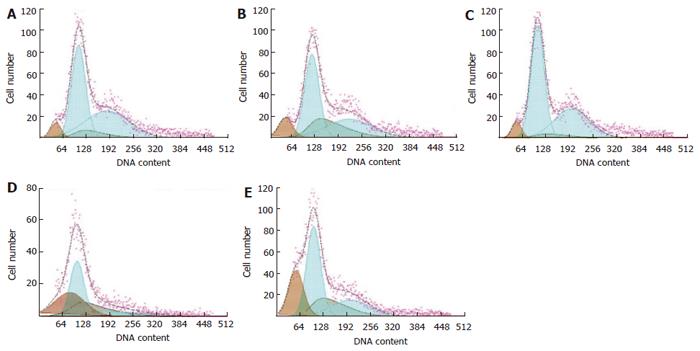
Fresh tumor tissue separated from nude mice was chopped with mechanical method. Single cell-suspended solution was obtained with 200 filter screens. The suspensions were divided into two parts. One part was fixed with 70% ice-cold ethanol for at least 12 h at 4 °C and then washed with PBS again before examination. The fixed cells were treated with RNase A and stained with PI at 37 °C for 30 min. Analysis was performed with a Coulter FCM (EPICS-XL) and data were analyzed with Multicycle DNA content and cell analysis software.
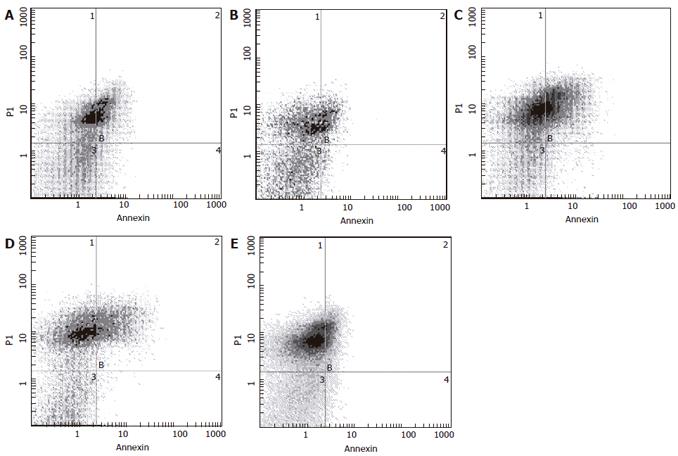
The Annexin V FITC kit (Jingmei Company, Lot No. 20050910) was used for detecting apoptosis according to manufacturer’s instructions. The suspensions were washed twice and adjusted to a concentration of 1 × 106 cells/mL with ice cold 4 °C PBS. The Falcon tubes (12 mm × 75 mm, polystyrene round-bottom) were used in experiment, 100 μL of suspensions was added to each labeled tube, 10 μL of Annexin V-FITC and 10 μL 20 μg/mL PI were added into the labeled tube, incubated for at least 20 min at room temperature in the dark, then 400 μL of PBS binding buffer was added to each tube without washing and analyzed by FCM as soon as possible (within 1 h).
Data were presented as mean ± SD and analyzed with SPSS 11.0 software. The analysis of variance (ANOVA) was used and least significant difference t test (LSD-t) was used for group comparison. P < 0.05 was considered statistically significant.
JLS granule at the doses of 20 g/kg, 60 g/kg and 120 g/kg significantly inhibited the growth of S180 subcutaneous tumor(P < 0.01) in a dose-dependent manner, but lower than the inhibitory rates of Cy (P < 0.05) (Table 1).
JLS granule at the doses of 20 g/kg, 60 g/kg and 120 g/kg significantly inhibited the growth of orthotopic-transplanted MKN-45 gastric cancer(P < 0.01) in a dose-dependent manner, but no difference statistically compared with 5-FU (P > 0.05 ) (Table 2).
| Group | n | Tumor weight (g) | Inhibition rate (%) |
| Control | 10 | 3.20 ± 0.96 | - |
| 120 g/kg | 10 | 1.02 ± 0.57b | 68.13 |
| 60 g/kg | 10 | 1.41 ± 1.39b | 55.94 |
| 20 g/kg | 10 | 1.59 ± 1.12b | 50.31 |
| 5-FU | 8 | 1.49 ± 1.37b | 53.43 |
Condensation of chromatin at margins of nuclei, disintegration of nucleolus, vacuoles in cytoplasm were observed in 60 g/kg group and 5-FU also had the same apoptotic appearance under TEM (Figure 1).
The percentage of G0/G1 cells in JLS granules group was higher than that in NS group and 5-FU group in a dose-dependent manner (P > 0.05 ). The cell percentage of S phase in of JLS group and 5-FU group was lower than that in control group (P < 0.05), the apoptotic rate in 120 g/kg JLS group was higher than that in control group, 20 g/kg JLS group and 5-FU group (P < 0.05). The typical apoptotic peak was observed (Table 3, Figure 2).
| Group | n | G0/G1 | G2/M | S | Apoptotic rate |
| Control | 10 | 42.40 ± 11.28 | 21.28 ± 11.43 | 36.92 ± 12.21 | 19.88 ± 14.83c |
| 120 g/kg | 10 | 50.58 ± 8.78 | 26.45 ± 12.53 | 14.56 ± 9.35a | 38.54 ± 12.65a |
| 60 g/kg | 10 | 47.55 ± 7.02 | 26.51 ± 15.05 | 19.36 ± 12.69a | 28.27 ± 11.25 |
| 20 g/kg | 10 | 46.37 ± 12.09 | 25.47 ± 14.79 | 24.04 ± 13.27a | 22.81 ± 10.65c |
| 5-FU | 8 | 41.78 ± 4.30 | 23.59 ± 11.97 | 19.97 ± 13.31a | 24.97 ± 9.93c |
Results by Annexin V-FITC/PI double staining for FCM assay showed that the early apoptotic cells were 4.36%, 3.08% and 7.08% in the three JLS granules groups (Figure 3), and most of cell populations in 5-FU treated group were located in the up right quadrant and the cells on the lower right quadrant were not many as compared with JLS granules groups.
Tumorigenesis, in a sense, is due to the deregulation in cell proliferation and apoptosis[13-14], accordingly tumor treatment not only inhibits cell proliferation, but also induces apoptosis. More and more attention is paid to apoptosis in treatment of tumors and the effectiveness of anti-tumor agents is often evaluated[15,16]. Investigators have confirmed that many Chinese herbs have antitumor property and induction of apoptosis is one of the mechanisms[17-20]. But most of these studies aimed directly at single herbs, bioactive constituents or leading compounds extracted from herbs, and could not educe the multi-target action of Chinese compounds and traditional Chinese medicine (TCM) theories in disease therapy [21].
In TCM theories, “phlegm” is not only a pathologic metabolite, but also a causative agent. The metabolites such as ectopic hormone, growth factor and tumor markers produced by tumor cells all belong to “phlegm”, which can devitalize physiologic functions and stimulate tumor invasion and metastasis in turn. Therefore, “dispersing phlegm and eliminating stagnation” (cleaning metabolites produced by tumor) is one of the regimens for tumor treatment in TCM. JLS granules are an anti-tumor compound prescription developed according to the methods of “dispersing phlegm and eliminating stagnation”. Our previous experiments have shown that “dispersing phlegm and eliminating stagnation” inhibit could VEGF, Ras mRNA and protein expression[22-24] and have good antitumor and anti-metastasis potency in vivo. However whether it induces apoptosis is unclear. Our studies showed that JLS granules had anti-tumor effects on S180 and MKN-45 human gastric cancer. The ultra-structure of MKN-45 gastric cancer tissue treated with JLS granules showed that there were apoptosis changes. Cell cycle and apoptosis analysis also demonstrated that JLS granules could arrest MKN-45 cells at G0/G1 phase, decrease S phase cell populations. The typical apoptosis peak further confirmed its apoptosis-inducing effect.
In addition, changes in the plasma membrane are one of the earliest morphological features of apoptosis. At this time, the membrane phospholipid phosphatidylserine (PS) is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external cellular environment. Annexin V is a 35-36kD Ca2+-dependent phospholipid binding protein that has a high affinity for PS, thus Annexin V staining serves as a sensitive probe for flow cytometric analysis of cells undergoing apoptosis[25-26]. Therefore, staining with Annexin V in conjunction with vital dyes such as propidiumiodide (PI) can identify early apoptotic cells (Annexin V positive, PI negative). In our study, cells treated with JLS granules were located in the low right quadrant and cells in 5-FU-treated group were located in the upper left quadrant, suggesting that the earlier apoptotic cells in JLS granules-treated groups are higher than those in 5-FU-treated group and that JLS granules induce apoptosis in human gastric cancer MKN-45 cells.
In conclusion, JLS granules have the inhibiting effects on human gastric carcinoma and induction of apoptosis may be one of its mechanisms.
The authors thank the Department of Laboratory Diagnosis, Changzheng Hospital, Shanghai, and Ling-Zhen Zhang for help in FCM assay.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 465] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27. [PubMed] [Cited in This Article: ] |
| 3. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 546] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 4. | Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. [1990-1992 mortality of stomach cancer in China]. Zhonghua Zhongliu Zazhi. 2002;24:4-8. [PubMed] [Cited in This Article: ] |
| 5. | Ushijima T, Sasako M. Focus on gastric cancer. Cancer Cell. 2004;5:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, McWilliam C, Gavin A, Baron RA, Aaron D. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18:2515-2521. [PubMed] [Cited in This Article: ] |
| 7. | Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14 Suppl 2:ii31-ii36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Wei Pk, Xu L, Qin ZF, Zhang S, Li XY, Guo XD, Wang JP, Li J, Xiao Y, Shi J. Treatment Mechanism of Phlegm and Clinical Research on Stomach Cancer. Zhongguo Zhongyi Jichu Yixue Zazhi. 2002;3:18-20. [Cited in This Article: ] |
| 9. | Xu L, Chen YL, Su XM, Wei PK. Study on Nude Mouse Model of Human Gastric Carcinoma Constructed by Using Orthotopic Transplantation and Their Biological Properties. Zhongliu Fangzhi Zazhi. 2003;10:476-478. [Cited in This Article: ] |
| 10. | Chen YL, Wei PK, Xu L, Su XM. [Nude mouse model of human gastric carcinoma metastasis constructed by orthotopic transplantation using organism glue paste technique]. Ai Zheng. 2005;24:246-248. [PubMed] [Cited in This Article: ] |
| 11. | Xiaoguang C, Hongyan L, Xiaohong L, Zhaodi F, Yan L, Lihua T, Rui H. Cancer chemopreventive and therapeutic activities of red ginseng. J Ethnopharmacol. 1998;60:71-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Han R. Research and Development of Anticancer Drugs and Experimental Techniques. Beijing: The United Press of Beijing Medical University and Peking Union Medical College 1997; 295-298. [Cited in This Article: ] |
| 13. | Kamesaki H. Mechanisms involved in chemotherapy-induced apoptosis and their implications in cancer chemotherapy. Int J Hematol. 1998;68:29-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2207] [Cited by in F6Publishing: 2312] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 15. | Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 385] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 16. | Alison MR, Sarraf CE. Apoptosis: regulation and relevance to toxicology. Hum Exp Toxicol. 1995;14:234-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Zhao HL, Zhao AG, You SF, Gu Y, Tang LD, Yang JK. [Growth-inhibiting and anti-metastasis effects of Weichang'an Decoction on orthotopic transplant nude mouse model of human gastric cancer]. Zhongxiyi Jiehe Xuebao. 2005;3:378-381. [PubMed] [Cited in This Article: ] |
| 18. | Liu W, Guo QL, You QD, Zhao L, Gu HY, Yuan ST. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J Gastroenterol. 2005;11:3655-3659. [PubMed] [Cited in This Article: ] |
| 19. | Yin X, Zhou J, Jie C, Xing D, Zhang Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004;75:2233-2244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Shao QS, Ye ZY, Ling ZQ, Ke JJ. Cell cycle arrest and apoptotic cell death in cultured human gastric carcinoma cells mediated by arsenic trioxide. World J Gastroenterol. 2005;11:3451-3456. [PubMed] [Cited in This Article: ] |
| 21. | Wang J, Jing L. [Study of Chinese herbs and its compound prescription guided by traditional Chinese medical theory]. Zhongguo Zhongyao Zazhi. 2003;28:795-798. [PubMed] [Cited in This Article: ] |
| 22. | Chen YL, Wei PK, Xu L, Su XM. Effects of Xiaotan Sanjie Formula on Expression of Oncogenes ras and cerb B2 at mRNA Level in Tissue of Gastric Carcinoma. Zhongyi Zazhi. 2004;45:459-461. [Cited in This Article: ] |
| 23. | Xu L, Wei PK, Chen YL, Su XM, Qin ZF, Shi J, Li J, He J. Xiaotansanjie recipe inhibits growth and metastasis of human gastric adenocarcinoma cell SGC-7901 transplanted in nude mouse. Shijie Huaren Xiaohua Zazhi. 2004;12:1015-1020. [Cited in This Article: ] |
| 24. | Xu L, Su XM, Chen YL, Wei PK. The effect of Xiaotansanjie recipe on VEGF and KDR mRNA expression of human gastric adenocarcinoma transplanted in nude mouse. Shijie Huaren Xiaohua Zazhi. 2004;12:988-990. [Cited in This Article: ] |
| 25. | Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3792] [Cited by in F6Publishing: 3909] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 26. | van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |