Published online Oct 7, 2005. doi: 10.3748/wjg.v11.i37.5882
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: October 7, 2005
AIM: To investigate the efficacy of combination treatment of IFN-α and lamivudine compared to lamivudine monotherapy, after 24 mo of administration in HBeAg-negative hepatitis B patients.
METHODS: Fifty consecutive patients were randomly assigned to receive IFN-α-2b (5 MU thrice per week, n = 24) plus lamivudine (100 mg daily) or lamivudine only (n = 26) for 24 mo. Patients were followed up for further 6 mo. The primary outcome was the proportion with sustained virological response (undetectable serum HBV DNA concentrations) and or sustained biochemical response (transaminase levels within normal range) at 30 mo (6 mo after the end of therapy). Secondary end-points were timed from initial virological (biochemical) response to VBR (BBR, respectively) and the emergence of YMDD mutants across the two arms.
RESULTS: Five of twenty-four (21%) patients in the combination arm vs 3/26 (12%) in the lamivudine arm had sustained response (i.e., normal serum transaminase levels and undetectable HBV DNA by PCR assay) 6 mo after treatment discontinuation. A reduction in the emergence of YMDD mutants and in the development of virological breakthroughs was observed in patients receiving combination treatment (10% vs 46%, P = 0.01 and 14% vs 46%, P = 0.03, respectively). Time from initial virologic response to virologic breakthrough (VBR) was greater among initial responders receiving combination treatment compared to those receiving lamivudine (22.9 mo vs 15.9 mo, respectively; P = 0.005).
CONCLUSION: Our results demonstrate that IFN-α plus lamivudine combination therapy does not increase the sustained response, compared to lamivudine. However, combination therapy reduces the likelihood of VBR due to YMDD mutants and prolongs the time period until the breakthrough development.
-
Citation: Economou M, Manolakopoulos S, Trikalinos TA, Filis S, Bethanis S, Tzourmakliotis D, Avgerinos A, Raptis S, Tsianos EV. Interferon-α plus lamivudine
vs lamivudine reduces breakthroughs, but does not affect sustained response in HBeAg negative chronic hepatitis B. World J Gastroenterol 2005; 11(37): 5882-5887 - URL: https://www.wjgnet.com/1007-9327/full/v11/i37/5882.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i37.5882
The HBeAg negative form of chronic hepatitis B (CHB) predominates in the Mediterranean area and has a rising frequency in Europe and North America. The HBeAg negative CHB is characterized by frequent exacerbation of hepatitis, which probably increases the risk of cirrhosis, hepatocellular failure, and hepatocellular carcinoma[1].
At present there are three approved therapies for patients with CHB: Interferon-α (IFN-α), lamivudine, and adefovir dipivoxil. Unfortunately, none of these drugs are effective in achieving a sustained response in patients with HBeAg negative CHB[2-6]. Therefore, new therapeutic approaches have been examined. Combination of antiviral drugs with different mechanisms of action or prolongation of treatment periods[7-10] have been reported with controversial results[11,12]. The aim of this study was to investigate the efficacy of combination treatment of IFN-α and lamivudine compared to lamivudine monotherapy, after 24 mo of administration in HBeAg-negative hepatitis B patients.
This was a multi-center open-labeled randomized controlled clinical trial, comparing IFN-α-2b 5 MU (Shering-Plough, Athens, Greece) t.i.w. plus oral lamivudine (GSK, Athens, Greece) 100 mg/d vs oral lamivudine 100 mg/d, for 24 mo. Random-ization was performed centrally, using lottery cards.
Between January 2000 and May 2001, 50 consecutive adult patients with HBeAg-negative CHB fulfilling all the inclusion criteria were enrolled in the study. Adult patients were eligible if they met all the inclusion criteria: (1) HBsAg positive, anti-HBe positive and HBeAg negative serology for at least 6 mo before enrollment, (2) serum HBV DNA concentrations >105 copies/mL, (3) elevated alanine transa-minase (ALT) activity (at least 1.5 times the upper normal limit) in three separate monthly occasions within the last 6 mo before randomization and (4) liver biopsy with evidence of chronic hepatitis within 12 mo before entering the study. The last patients enrolled ended their follow-up in December 2003.
Patients were excluded if they had antibodies against HIV or hepatitis C or D viruses, had decompensated liver disease (Child-Pugh score >8) or had previously received liver transplantation. We also excluded patients who had been treated with any antiviral drug other than IFN and those who had received immunosuppressive therapy within 6 mo before participation. Patients with active alcohol consumption (>50 g/d) or suspected hepatocellular carcinoma (by real-time ultrasonography and elevated a-fetoprotein) were also excluded. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and all patients gave informed consent.
Clinical examination and routine laboratory tests were performed every 2 wk during the first 3 mo of treatment and every month thereafter. Compliance was monitored at each visit by assessing the number of used drugs. Laboratory tests included complete blood count, ALT, aspartate transaminase (AST), amylase, alkaline phosphatase, g-glutamyl transpeptidase, creatinine, and prothrombin time. Serum HBV DNA and a-fetoprotein were evaluated every 6 mo, until the end of the follow-up period (30 mo). Potential side effects were recorded at each visit. All treated patients were followed up for additional 6 mo after they stopped their treatment.
We regarded as initial virologic response the clearance of serum HBV DNA by PCR, and as initial biochemical response the decline of transaminase levels to normal in two consecutive determinations. We considered as virologic breakthrough (VBR) the reappearance of detectable serum HBV DNA by PCR after an initial virologic response, and as biochemical breakthrough (BBR) the increase of ALT or AST levels to greater than 1.5 times the upper normal limit after an initial biochemical response[13]. The primary outcome was the proportion with sustained virological response (undetectable serum HBV DNA concentrations) and/or sustained biochemical response (transaminase levels within normal range) at 30 mo (6 mo after the end of therapy). Secondary end-points were timed from initial virological (biochemical) response to VBR (BBR, respectively) and the emergence of YMDD mutants across the two arms.
Serum ALT, AST, albumin, bilirubin, prothrombin time, and a-fetoprotein levels were measured by standard laboratory procedures. HBsAg, HBeAg, anti-HBe, anti-HBc, anti-HCV, anti-HDV, and anti-HIV titers were measured using commercially available microparticle enzyme immunoassays. Serum HBV DNA viral load levels were determined using AMPLICOR HBV MONITOR (Roche Diagnostics Systems, NJ, USA). The test incorporates a known amount of synthetic internal standard (IS), which is co-amplified. After amplification, a colorimetric reaction determines the amplified products. The HBV/IS ratio for each test specimen is compared against a standard curve and the HBV DNA viral load of the tested samples is calculated from the standard curve in copies per milliliter. The detection limit of this assay according to the manufacturer is 400 copies/mL[14].
YMDD mutants were evaluated at baseline and on any occasion of VBR. The YMDD motif of tested serum samples was determined by a line probe assay (INNO-LIPA HBV DR, Innogenetics NV, Belgium). DNA extraction, subsequent HBV polymerase region amplification, detection, and readout were performed according to the manufacturer’s instructions[15].
The analyses were performed according to the intention-to-treat (ITT) principle, considering dropouts as failures for the primary endpoint. We used the last observation carried forward (LOCF) approach, where the last available measurement is imputed for missing values. We also performed secondary analyses excluding dropouts (“per protocol” analyses). We compared baseline characteristics between the two arms using Mann-Whitney or c2 tests, as appropriate. Dropouts across the two arms were compared with Fisher’s exact test. HBV DNA values below detection (<400 copies/mL) were imputed to 399 copies/mL for the analyses. HBV DNA concentrations were log-transformed for normality. Univariate and multivariate logistic regressions were used to address possible relationships between baseline characteristics and the primary outcome. Time from initial virologic (or biochemical) response to VBR (or BBR, respectively) as well as time to YMDD emergence were explored with Kaplan-Meier analyses and multivariate Cox proportional hazards models. In all multivariate analyses (logistic regressions and Cox models), we considered as covariates the treatment allocation arm, age, gender, baseline transaminase level, baseline logarithm of HBV DNA, prior IFN therapy, histology activity index (appropriately coded with dummy variables), and presence of cirrhosis at baseline. Selection of covariates for the final models was performed with backward elimination based on likelihood ratio criteria. Serial measurements of ALT, AST, and log HBV DNA concentrations were compared employing repeated measures analysis of variance, using the Huynh-Feldt adjustment whenever sphericity assumptions were violated. P values < 0.05 were considered as statistically significant. All P-values were two-tailed. Analyses were performed in SPSS 10.0 (SPSS Inc., Chicago, IL, USA) and STATA 8.2 (STATA Corp., College Station, TX, USA).
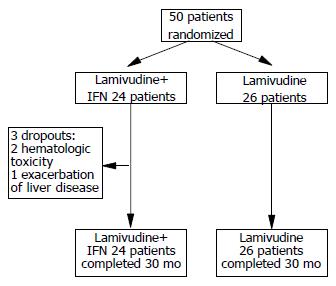
During study period, 50 consecutive adult patients with HBeAg-negative CHB fulfilling all the inclusion criteria were randomly assigned to lamivudine plus IFN (n = 24) and to lamivudine monotherapy (n = 26, Figure 1). The baseline characteristics of the two arms were similar with respect to demographic and clinical features (Table 1). Twenty-eight patients had previously been treated with IFN-α: 6 had not responded and 22 had relapsed after discontinuing treatment. Three patients in the lamivudine plus IFN-α arm vs none in the lamivudine arm dropped out by the 10th wk (exact P = 0.10). None of the patients were dropped due to protocol violations.
| IFN-α+lamivudine(n = 24) | Lamivudine(n = 26) | P | |
| Age (median, IQR) | 53 (47, 60) | 58 (41, 66) | 0.36 |
| Male (%) | 15/24 (63) | 18/26 (69) | 0.62 |
| ALT, median (IQR) | 79 (57, 100) | 59 (52, 94) | 0.24 |
| AST, median (IQR) | 59 (51, 89) | 60 (40, 94) | 0.43 |
| log10(HBV), median (IQR) | 6.1 (5.2, 7.2) | 5.9 (5.2, 6.6) | 0.72 |
| Previous IFN-α therapy (%) | 13/24 (54) | 15/26 (58) | 0.80 |
| Cirrhosis1 (%) | 11/24 (45.8) | 13/26 (50) | 0.7 |
In ITT analyses, 5/24 (21%) patients in the IFN-α plus lamivudine arm vs 3/26 (12%) patients in the lamivudine monotherapy arm had sustained response to therapy at 30 mo. Hence, the combination therapy was not more likely to lead to sustained response at the end of the follow-up period (rate ratio, RR = 1.81 [95%CI: 0.48-6.76]). The results were essentially the same for per protocol analyses (RR = 2.06 [95%CI: 0.56-7.65]). None of the patients cleared HBsAg at 30-mo study period. Univariate and multivariate logistic regression analyses did not identify a statistically significant relationship between any of the baseline characteristics and sustained response at 30 mo.
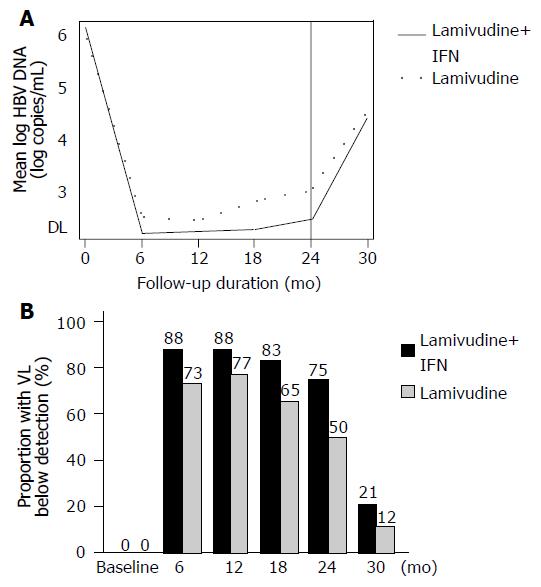
The mean log HBV DNA was not different across the two arms over time (P = 0.26; Figure 2A). The proportion of patients with serum HBV DNA below detection by ITT-LOCF for every assessment is shown in Figure 2B. In per protocol analyses, the proportion of patients who lowered their viral concentrations below detection was 21/21 = 100% vs 19/26 = 73% at 6 mo; 21/21 = 100% vs 20/26 = 77% at 12 mo; 20/21 = 95% vs 17/26 = 65% at 18 mo; and 18/21 = 86% vs 13/26 = 50% at 24 mo of treatment, between combination and monotherapy, respectively. In the combination arm, all patients except for the three dropouts had an initial virologic response during the first 6 mo of therapy. In the lamivudine arm, an initial virologic response was observed in 24/26 (92%) within the first 12 mo (19 patients by 6 mo, and 5 patients by 12 mo). The remaining two patients in the lamivudine group did not clear HBV DNA; however, their viral load concentrations dropped by at least 2 log during the first 12 mo of treatment.
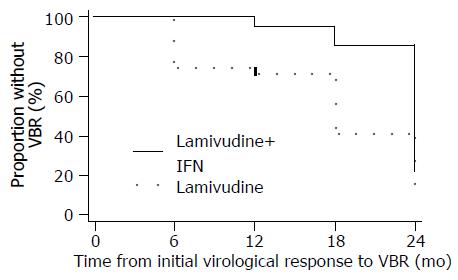
During 24 mo therapy, VBR were observed in 3 (14%) of the 21 patients with an initial virological response in the combination arm and in 11 (46%) of the 24 patients in the lamivudine arm respectively (P = 0.03). Time from initial virologic response to VBR was significantly greater among the 21 initial responders in the IFN-α plus lamivudine arm compared to the 24 initial responders in the lamivudine arm (Figure 3; log-rank P = 0.005). The mean time to VBR was 22.9 mo (95%CI: 21.5-24.2) vs 15.9 mo (95%CI: 12.7-19.2), respectively. At the onset of VBR, median serum HBV DNA concentrations were similar between the two groups (P = 0.10). Furthermore at the onset of VBR, serum HBV DNA concentrations exceeded 100 000 copies/mL in only two patients, but became greater than 100 000 copies/mL in five more patients with at least 6 mo treatment after VBR.
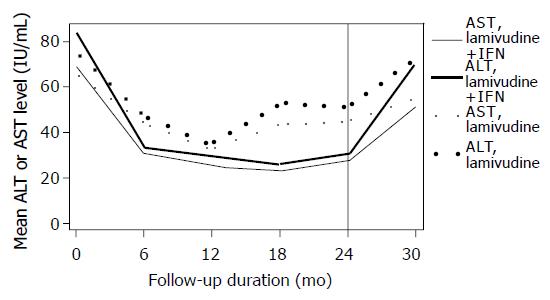
Median ALT and AST values were statistically significantly lower in the IFN-α plus lamivudine arm compared to lamivudine monotherapy during the treatment period (P = 0.04; Figure 4). The proportion of patients with transaminase levels within normal range (per protocol analyses) was 14/21 = 67% vs 12/26 = 46% at 6 mo, 18/21 = 86% vs 21/26 = 81% at 12 mo, 19/21 = 90% vs 19/26 = 73% at 18 mo, 19/21 = 90% vs 16/26 = 62% at 24 mo and 6/21 = 28% vs 5/26 = 19% at 30 mo of follow-up for the two arms, respectively. All patients who cleared the serum HBV DNA at 30 mo had normal transaminase levels (complete response).
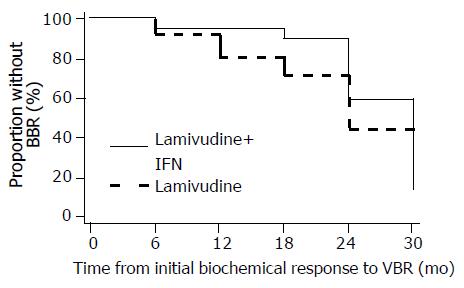
BBR was developed in seven patients with an initial biochemical response, two in the combination arm and five in the lamivudine arm respectively. None of the patients with BBR in both groups developed clinical signs of decompensation.At the onset of BBR, transaminase level exceeded 400 IU/L (> 10 times the upper normal limit) in two patients receiving lamivudine monotherapy. Elevation of bilirubin to abnormal levels was observed in one of these patients. During BBR period, transaminase levels remained abnormal in all patients. Lamivudine therapy was continued in all patients with BBR in both groups. None of the patients with BBR received other anti-viral treatment. Combination therapy tended to delay BBR for a longer time period after the initial biochemical response, compared to those on lamivudine only. However, this was not formally statistically significant (Figure 5; log-rank P = 0.18). Similarly, time from initial biochemical response to BBR was not significantly dependent on any of the covariates in the multivariate Cox models.
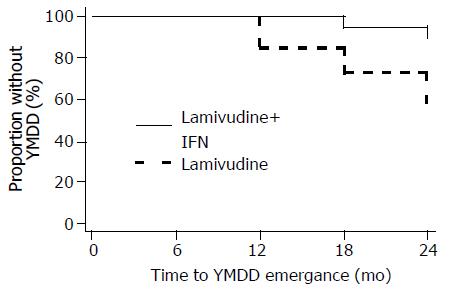
None of the patients had YMDD mutants at baseline. YMDD mutations were detected in two of the three patients with VBR in the combination arm (one had the M552I mutant and the other the L528M+M552I mutant strains). In the lamivudine arm, YMDD mutants were detected in all 11 patients with VBR (three patients with VBR had the L528M+M552V mutant; three the L528M+M552I; two the M552I; and three the M552V mutant strains) and in one of the two patients who had detectable serum HBV DNA at all measurements (L528M+M552I mutant strains). Overall, the frequency of emergence of treatment of YMDD mutations in this study was 2/21 (10%) in the IFN-α plus lamivudine arm and 12/26 (46%) in the lamivudine arm (exact P = 0.01). The mean time to YMDD emergence was significantly longer in the combination arm compared to the monotherapy arm (23.7 mo [95%CI: 22.9-24.5] vs 21.5 mo [95%CI: 19.7-23.3], respectively, Figure 6; log-rank P = 0.013).
|Three patients in the IFN-α plus lamivudine arm discontinued IFN-α because of hematologic toxicity in two cases and severe exacerbation of liver disease in one case. All 21 remaining patients in this arm reported minor side-effects. All patients tolerated lamivudine monotherapy very well throughout the study period.
Our results confirmed our initial hypothesis. The data presented clearly suggest that 2 years treatment with the combination of IFN-α plus lamivudine induces higher rates of biochemical and virological response, reduces the possibility of YMDD mutations and delays the development of VBR in patients with HBeAg negative form of CHB.
This is the first study to suggest that the combination of IFN-α plus lamivudine may reduce and delay the VBR phenomenon during the treatment period. The only available evidence that this might happen comes from studies showing that the combination treatment is associated with a reduction in YMDD emergence[8-10] independently of HBeAg status. In accordance with these studies, we have also found a similar reduction in escape YMDD mutants. However, none of these studies have shown that this might be followed with a difference in VBR. The explanation might be attributed to the longer duration of treatment in our group of patients. As shown, the difference between the two groups of our patients became evident after 12 mo of continuous treatment. In favor of the above hypothesis is also the fact that in none of the studies conducted so far patients have been treated with the combination of IFN-α with lamivudine for a period longer than 12 mo.
It has been shown that the VBR phenomenon is usually associated with treatment failure[16,17]. It has therefore been anticipated that the reduction in the VBR may be associated with an increase in the number of patients with sustained response to treatment. However, we did not observe a difference in sustained response between the two groups of patients studied. According to our results, any kind of prolongation of remission does not appear to secure sustained remission of the disease. The reason of this phenomenon is difficult to understand. Perhaps in HBeAg negative form of CHB, the longer duration of infection is associated with a higher number of infected hepatocytes which may need longer duration of treatment in order to eradicate the virus[18]. Another possible explanation might be that interferon is not enough to restore the immune responsiveness.
We have identified a tendency in favor of the combination arm regarding sustained response rates at 6 months of follow-up, which did not reach formal statistical significance. If one synthesized findings from the other two relevant controlled trials[19,20] with the results from our trial in a formal meta-analysis, the summary sustained response rate ratio would be 30% greater for the combination arm (rate ratio = 1.28 [95%CI: 0.75-2.19], both by fixed and random effects meta-analysis[21] with no statistical dissimilarity -heterogeneity-across synthesized trials). The summary effect size cannot exclude a twofold difference in the sustained response rate between the two arms. This merely reflects the fact that all relevant trials are underpowered to detect the a posteriori observed differences across the two arms: a trial would have to randomize 440 people in order to be able to detect with 80% power, a difference of 20% vs 10% at the 0.05 significance level. For example, an adequately powered trial by Marcellin et al[22], which examined the efficacy of the Pegylated-IFN-α2a in 537 patients with HBeAg negative CHB, was able to identify that the Pegylated IFN-α-2a/lamivudine combination was significantly superior to lamivudine and not different than Pegylated-IFN-α-2a monotherapy.
In our study, we used a sensitive PCR assay for HBV DNA determinations in contrast to less sensitive hybridization assays which were used by previous investigators. At the onset of VBR, only two patients had serum HBV DNA concentrations above 100 000 copies/mL. However, serum HBV DNA increased to levels greater than 100 000 copies/mL in five more patients during the treatment period. Our data are in accordance with those reported by Papatheodoridis et al[16], who observed that only 2/32 patients had HBV DNA concentrations greater than 100 000 copies/mL at the onset of the breakthrough. Therefore, a close serum HBV DNA monitoring by a sensitive PCR assay is necessary in order to recognize the VBR early.
In conclusion, our study clearly shows that the combination of IFN-α plus lamivudine reduces the YMDD mutants and delays VBR, but does not affect significantly the sustained response in HBeAg negative CHB as compared to lamivudine monotherapy. Further well-designed studies are also urgently required in order to explore the mechanism involved in achieving a sustained response in this difficult-to-treat group of patients.
We thank Mrs. P. Georgitsi and Mr. G. Filis for their technical assistance in the genotypic analyses.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Manesis EK, Hadziyannis SJ. Interferon alpha treatment and retreatment of hepatitis B e antigen-negative chronic hepatitis B. Gastroenterology. 2001;121:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, Hawley S, Barber J, Condreay L, Gray DF. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology. 1999;29:889-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 376] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 4. | Santantonio T, Mazzola M, Iacovazzi T, Miglietta A, Guastadisegni A, Pastore G. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine. J Hepatol. 2000;32:300-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Hadziyannis SJ, Papatheodoridis GV. Treatment of HBeAg negative chronic hepatitis B with new drugs (adefovir and others). J Hepatol. 2003;39 Suppl 1:S172-S176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Wong DK, Cheung AM, O'Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 746] [Cited by in F6Publishing: 742] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 7. | Mutimer D, Dowling D, Cane P, Ratcliffe D, Tang H, O'Donnell K, Shaw J, Elias E, Pillay D. Additive antiviral effects of lamivudine and alpha-interferon in chronic hepatitis B infection. Antivir Ther. 2000;5:273-277. [PubMed] [Cited in This Article: ] |
| 8. | Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, Dhillon A, Moorat A, Barber J, Gray DF. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:562-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Barbaro G, Zechini F, Pellicelli AM, Francavilla R, Scotto G, Bacca D, Bruno M, Babudieri S, Annese M, Matarazzo F. Lamivudine Italian Study Group Investigators. Long-term efficacy of interferon alpha-2b and lamivudine in combination compared to lamivudine monotherapy in patients with chronic hepatitis B. An Italian multicenter, randomized trial. J Hepatol. 2001;35:406-411. [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Schiff ER, Dienstag JL, Karayalcin S, Grimm IS, Perrillo RP, Husa P, de Man RA, Goodman Z, Condreay LD, Crowther LM. Lamivudine and 24 weeks of lamivudine/interferon combination therapy for hepatitis B e antigen-positive chronic hepatitis B in interferon nonresponders. J Hepatol. 2003;38:818-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Lampertico P, Del Ninno E, Manzin A, Donato MF, Rumi MG, Lunghi G, Morabito A, Clementi M, Colombo M. A randomized, controlled trial of a 24-month course of interferon alfa 2b in patients with chronic hepatitis B who had hepatitis B virus DNA without hepatitis B e antigen in serum. Hepatology. 1997;26:1621-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 133] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Janssen HL, Gerken G, Carreño V, Marcellin P, Naoumov NV, Craxi A, Ring-Larsen H, Kitis G, van Hattum J, de Vries RA. Interferon alfa for chronic hepatitis B infection: increased efficacy of prolonged treatment. The European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology. 1999;30:238-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Hadziyannis SJ, Papatheodoridis GV, Dimou E, Laras A, Papaioannou C. Efficacy of long-term lamivudine monotherapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2000;32:847-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 279] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Kessler HH, Pierer K, Dragon E, Lackner H, Santner B, Stünzner D, Stelzl E, Waitzl B, Marth E. Evaluation of a new assay for HBV DNA quantitation in patients with chronic hepatitis B. Clin Diagn Virol. 1998;9:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Stuyver L, Van Geyt C, De Gendt S, Van Reybroeck G, Zoulim F, Leroux-Roels G, Rossau R. Line probe assay for monitoring drug resistance in hepatitis B virus-infected patients during antiviral therapy. J Clin Microbiol. 2000;38:702-707. [PubMed] [Cited in This Article: ] |
| 16. | Papatheodoridis GV, Dimou E, Laras A, Papadimitropoulos V, Hadziyannis SJ. Course of virologic breakthroughs under long-term lamivudine in HBeAg-negative precore mutant HBV liver disease. Hepatology. 2002;36:219-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Manolakopoulos S, Karatapanis S, Elefsiniotis J, Mathou N, Vlachogiannakos J, Iliadou E, Kougioumtzan A, Economou M, Triantos C, Tzourmakliotis D. Clinical course of lamivudine monotherapy in patients with decompensated cirrhosis due to HBeAg negative chronic HBV infection. Am J Gastroenterol. 2004;99:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Hadziyannis SJ. Hepatitis B e antigen negative chronic hepatitis B: from clinical recognition to pathogenesis and treatment. Viral Hepat Rev. 1995;1:7-36. [Cited in This Article: ] |
| 19. | Jaboli MF, Fabbri C, Liva S, Azzaroli F, Nigro G, Giovanelli S, Ferrara F, Miracolo A, Marchetto S, Montagnani M. Long-term alpha interferon and lamivudine combination therapy in non-responder patients with anti-HBe-positive chronic hepatitis B: results of an open, controlled trial. World J Gastroenterol. 2003;9:1491-1495. [PubMed] [Cited in This Article: ] |
| 20. | Santantonio T, Niro GA, Sinisi E, Leandro G, Insalata M, Guastadisegni A, Facciorusso D, Gravinese E, Andriulli A, Pastore G. Lamivudine/interferon combination therapy in anti-HBe positive chronic hepatitis B patients: a controlled pilot study. J Hepatol. 2002;36:799-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1858] [Cited by in F6Publishing: 1940] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 22. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 874] [Cited by in F6Publishing: 802] [Article Influence: 40.1] [Reference Citation Analysis (0)] |