Published online Oct 7, 2005. doi: 10.3748/wjg.v11.i37.5757
Revised: February 23, 2005
Accepted: February 28, 2005
Published online: October 7, 2005
AIM: To explore whether polymorphisms of the CYPIA1 and GSTM1 genes are associated with susceptibility of stomach cancer.
METHODS: A total of 102 stomach cancer cases and 62 healthy persons were diagnosed by pathology in 1998-2000 in the Qilu Hospital of Shandong University. Gene polymorphisms were detected by the PCR using sequence-specific primers. Data analysis of the case-control study was carried out using the unconditional logistic method.
RESULTS: After adjustment for age, sex, educational levels, and occupation, the risk factors for stomach cancer were shown to be smoking, Helicobacter pylori (H pylori), and presence of the CYPIM G/G and GSTM1 O/O genotypes. Interaction was observed between the combined genotypes of either CYPIA1 G/G and GSTM1 O/O or H pylori infection, or GSTM1 O/O and H pylori infection or smoking.
CONCLUSION: Polymorphisms of the CYPIA1 and GSTM1 genes, H pylori infection and smoking are related to susce-ptibility to stomach cancer.
- Citation: Li H, Chen XL, Li HQ. Polymorphism of CYPIA1 and GSTM1 genes associated with susceptibility of gastric cancer in Shandong Province of China. World J Gastroenterol 2005; 11(37): 5757-5762
- URL: https://www.wjgnet.com/1007-9327/full/v11/i37/5757.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i37.5757
It has been found that the progression of precancerous lesions to gastric cancer is associated with cigarette smoking, alcohol drinking, and Helicobacter pylori (H pylori) infection in high-incidence areas of China[1-3].
Individual susceptibility to cancer may be partly due to exposure to environmental risk factors, which can be partly explained by genetic variability in metabolic activities related to phases I and II detoxification enzyme pathways. Polym-orphisms in these metabolic susceptible genes have been linked to increased risk of cancer in several case-control studies[4].
Cytochrome p450 enzymes, which represent a large multigene family with different substrate specificities, are important in phase I detoxification reactions. The CYPIA1 gene product, aromatic hydrocarbon hydroxylase, catalyzes the first oxidative step in the metabolism of polycyclic aromatic hydrocarbons, such as those found in tobacco smoke, to carcinogens. This gene is induced by exposure to agents such as dioxin, benzo [a] pyrene and other aromatic hydrocarbons[5].
Various CYPIA1 gene polymorphisms have been found to be differentially associated with increased risk of several cancers (including cancer of the lung, breast, and colon) in different specific ethnic groups[6-9].
Phase II enzymes, glutathione S-transferase (GSTs), also have activated metabolites of carcinogens to be subjected to metabolic conjugation and other kinds of detoxifications. Homozygous deletions or null genotypes of GSTT1 (theta class) and GSTM1 (mu class) genes may be associated with an increased risk of cancer[5,7-9].
The polymorphisms of these metabolic enzymes result from a difference of the genotypes. Some of these forms cannot metabolize carcinogens into non-cytotoxic agents as is observed in the wild-type proteins[10]. Otherwise some of them metabolize cytotoxic agents into compounds that have the potential to damage DNA associated with high susce-ptibility to many kinds of diseases or drug resistance[11].
Few studies have evaluated the relationship between CYPIA1, GSTM1, and the risk of gastric cancer, as well as the potential interactions between these genetic markers and other risk factors for gastric cancer in the Chinese population. The present study was to investigate the interaction between CYPIA1- and GST-susceptible genotypes of gastric cancer and H pylori infection or smoking.
Patients with stomach cancer were recruited between January 1998 and January 2000 in the Affiliated Hospital of Shandong University. A biopsy was taken under gastroscope from all patients for their pathologic diagnosis. Of these patients, 102 (86 males and 16 females) had stomach cancer as the case group, and 62 (33 males and 29 females) had normal gastrointestinal mucous membrane and were used as the control group. All subjects were interviewed using a standardized questionnaire concerning their education, occupation, and cigarette smoking, etc. Blood samples (5 mL) were obtained from patients and controls, and 0.5 mL anti-clot solution of ACD was added. DNA was isolated from peripheral WBC by digestion with proteinase K, followed by phenol/chloroform extraction, and ethanol precipitation prior to resuspension in buffer containing 0.05 mol/L Tris, 5 mol/L EDTA, pH 7.6.
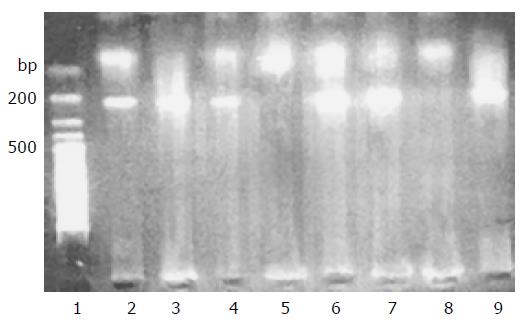
CYPIA1 genetic polymorphisms were determined as described previously[10]. Two pair primer sets were used to detect the polymorphism within exon 7 of the CYPIA1 gene by PCR. One pair primers were sense primer 1A1A: 5-GAAGTGTATCGGTGAGACCA-3 and antisense primer c53: 5-GTAGACAGAGTCTAGGCCTCA-3; the other pair primers were sense primer 1A1G: 5-GAAGT-GTATCGGTGAGACCG-3 and antisense primer c53: 5-GTAGACAGAGTCTAGGCCTCA-3 located about 190 bp downstream of the polymorphism site (Figure 1).
PCR was carried out in two tubes with a total volume of 30 mL containing 0.4 mg of genomic DNA, 1 mL of each of 1A1A and C53 primers 1A1G and C53 were added into the other tube, 2.5 mL of 10×PCR buffer, 2.5 mL of 25 mmol/L Mg2+, 1.5 mL of 10 mmol/L dNTP, 1 U Taq DNA polymerase. The program was initiated 8 min of denaturation at 95 °C, followed by 35 cycles of amplification with denaturation for 90 s at 95 °C, annealing for 60 s at 65 °C and an extension at 72 °C for 120 s, a final elongation at 72 °C for 6 min and 40 s on a water incubation PCR amplifier.
The PCR products were then subjected to electrophoresis on 1.8% agarose gels. If there was only a faint band of 190 bp in the 1A1A tube, the genotype was recorded as A/A. If there was only a faint band in the 1A1G tube, the genotype was determined to be G/G.
If both 1A1A and 1A1G tubes showed the faint bands, the genotype was recorded as A/G.
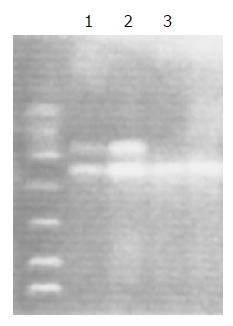
Two primers (G5: 5-GAACTCCCTGAAAAGCTA-AAGC-3 and G6: 5-GTTGGGCTCAAATATACGG-TGG-3) and the primer of b-protein (5-CAACTTCATC-CACGTTCACC-3 and 5-GAAGAGCCAAGGACA-GGTAC-3), which is located about 215 and 286 bp, downstream of the polymorphic sites, were used to deter-mine the GSTM1 genetic polymorphism as previously described[11](Figure 2).
PCR were carried out in two tubes of a total volume of 30 mL containing 0.4 mg of genomic DNA, 0.5 mL of each primer, 3.0 mL of 10×PCR buffer, 2.5 mL of 25 mmol/L Mg2+, 3.0 mL of 10 mmol/L dNTP, 2 U of Taq DNA polymerase. The program was initiated for 4 min of denaturation at 94 °C, followed by 30 cycles of amplification with denaturation for 30 s at 94 °C, annealing for 30 s at 57 °C and an extension at 72 °C for 45 s, a final elongation at 72 °C for 5 min on a Perkin Elmer PCR amplifier.
The PCR products were then subjected to electrophoresis on 1.8% agarose gels. If there were light intensity bands at 215 and 268 bp, the genotype was recorded as +/+. If there was only a light band of 268 bp, the genotype was determined to be O/O. If there was a faint band of 215 bp and a more intense band of 268 bp, the genotype was recorded as +/O.
All subjects were tested with an antibody against H pylori for evidence of infection using an ELISA kit.
To examine the association between CYPIA1 and GSTM1 genotypes and stomach cancer, we calculated the odds ratio (OR) and 95% confidence interval (CI) by unconditional logistic regression. The ORs were adjusted for potential confounding factors, including age, sex, education, and occupation.
Possible modification of the association between genetic polymorphism and stomach cancer risk was also evaluated by stratifying cases and controls into tertiles based on H pylori infection and smoking.
We initially assessed the risk of stomach cancer using a variety of parameters. As reported previously, both smoking and H pylori infection led to a significant increase in risk (Table 1).
| Variable | Value level | Gastric group | Control group | χ2 | P | ||
| n | % | n | % | ||||
| Age (yr) | <35 | 4 | 3.9 | 18 | 29 | ||
| 35- | 13 | 12.7 | 21 | 33.9 | |||
| 45- | 85 | 83.3 | 23 | 37.1 | 38.945 | <0.001 | |
| Sex | Male | 86 | 84.3 | 33 | 53.2 | ||
| Female | 16 | 15.7 | 29 | 46.8 | 18.718 | <0.001 | |
| Education years | <5 | 57 | 55.9 | 14 | 22.6 | ||
| 6- | 29 | 28.4 | 28 | 45.2 | |||
| 13- | 16 | 15.7 | 20 | 32.3 | 17.807 | <0.001 | |
| Occupation | Farmer | 63 | 61.8 | 24 | 38.7 | ||
| Worker | 21 | 20.6 | 18 | 29 | |||
| Card | 18 | 17.6 | 20 | 32.3 | 8.573 | 0.014 | |
There was no evidence for an increased risk of gastric cancer associated with sauerkraut and mildew food consumption or imbibing white spirit in Chinese population. However, frequent consumption of raw garlic was associated with an apparently reduced risk. The most significant factor associated with increased risk of stomach cancer was a CYPIA1 G/G genotype where the OR was 4.84 compared to the A/A and A/G genotypes. The GSTM1 O/O genotype was also associated with an increased risk of gastric cancer (Table 2).
| Factors | Controls | Cases | OR1 | 95%CI | OR2 | 95%CI | ||
| Chinese sauerkraut | ||||||||
| None | 53 | 95 | 1 | |||||
| Sometime | 6 | 4 | 0.37 | 0.08 | 1.58 | 0.24 | 0.04 | 0.95 |
| Often (2+/wk) | 3 | 3 | 0.56 | 0.09 | 3.62 | 0.39 | 0.41 | 3.3 |
| Mildew food | ||||||||
| None | 59 | 94 | 1 | |||||
| Sometime | 2 | 5 | 1.57 | 0.2 | 12.11 | 1.5 | 0.22 | 10.14 |
| Often (2+/mo) | 1 | 3 | 1.88 | 0.17 | 48.13 | 1 | 0.07 | 14.69 |
| Eat raw garlic/yr | ||||||||
| <1 000 | 20 | 77 | 1 | |||||
| >1 000 | 42 | 25 | 0.15 | 0.07 | 0.33 | 0.15 | 0.06 | 0.33 |
| CYPIA1 genotype | ||||||||
| A/A | 35 | 53 | 1 | |||||
| A/G | 24 | 27 | 0.74 | 0.35 | 1.58 | 0.59 | 0.26 | 1.34 |
| G/G | 3 | 22 | 4.84 | 1.24 | 22.07 | 5.91 | 1.28 | 27.24 |
| H pyloriinfection | ||||||||
| - | 26 | 13 | 1 | |||||
| + | 36 | 89 | 4.64 | 2.15 | 11.51 | 4 | 1.65 | 9.71 |
| Drinking white alcohol3 | ||||||||
| None | 37 | 48 | 1 | |||||
| 1-00 | 8 | 9 | 0.87 | 0.27 | 2.77 | 1.03 | 0.26 | 4.02 |
| 100+ | 17 | 45 | 2.04 | 0.95 | 4.39 | 1.11 | 0.41 | 2.99 |
| Smoking4 | ||||||||
| None | 42 | 46 | 1 | |||||
| <270 | 14 | 15 | 0.98 | 0.39 | 2.46 | 0.66 | 0.22 | 1.94 |
| ≥270 | 6 | 41 | 6.23 | 2.24 | 18.27 | 2.78 | 0.9 | 8.65 |
| History of cancer family | ||||||||
| None | 59 | 88 | 1 | |||||
| Yes | 3 | 14 | 3.13 | 0.79 | 14.38 | 2.98 | 0.69 | 12.82 |
| GSTM1 genotype | ||||||||
| +/+ or +/O | 36 | 33 | 1 | |||||
| O/O | 26 | 67 | 2.81 | 1.39 | 5.71 | 2.72 | 1.32 | 5.62 |
When the ORs were calculated for the combined CYPIA1 and GSTM1 genotypes, a combination of CYPIA1 A/A and GSTM1 +/+ or +/O gave a baseline of 1.0 as shown in Table 3. The OR for GSTM1 O/O genotype was 3.34 (95%CI: 1.13-9.87). The OR for CYPIA1 G/G was 4.16 (95%CI: 0.40-43.45). However, combined CYPIA1 G/G and GSTM1 O/O enhanced the risk of gastric cancer (OR = 16.48, 95%CI: 2.36-115.0). There was a significant interaction.
| CYPIA1 | GSTM1 | Controls | Cases | OR1 | 95%CI | |
| A/A | +/+ or+/O | 22 | 17 | 1.00 | ||
| O/O | 13 | 35 | 3.34 | 1.13 | 9.87 | |
| A/G | +/+ or+/O | 13 | 10 | 0.65 | 0.20 | 2.14 |
| O/O | 11 | 16 | 1.67 | 0.52 | 5.36 | |
| G/G | +/+ or+/O | 1 | 6 | 4.16 | 0.40 | 43.45 |
| O/O | 2 | 16 | 16.48 | 2.36 | 115.0 | |
When the ORs were calculated for the combined CYPIA1 genotypes and H pylori infection, a combination of CYPIA1 A/A and no H pylori infection at a baseline of 1.0, the OR of H pylori infection alone was 7.19 (95%CI: 1.98-26.11), and the OR of CYPIA1 G/G genotype alone was 13.18 (95%CI: 0.42-414.9). The OR significantly enhanced for the combined CYPIA1 G/G genotype and H pylori infection compared to the baseline group (Table 4).
| CYPIA1 | H pylori | Controls | Cases | OR1 | 95%CI | |
| A/A | - | 18 | 5 | 1.00 | ||
| + | 17 | 22 | 7.19 | 1.98 | 26.11 | |
| A/G | - | 7 | 5 | 1.59 | 0.29 | 8.91 |
| + | 17 | 48 | 2.94 | 0.78 | 11.13 | |
| G/G | - | 1 | 3 | 13.18 | 0.42 | 414.9 |
| + | 2 | 19 | 28.11 | 3.95 | 199.8 | |
As shown in Table 4, when compared to the combined CYPIA1 A/A genotype and non-smoking at a baseline of 1.0, the OR of smoking alone was 1.53 (95%CI: 0.47-4.96), and the OR of CYPIA1 G/G genotype alone was 4.90 (95%CI: 0.90-26.73), both of them did not reach a significant level. However, the ORs were significantly higher for the combined CYPIA1 G/G genotype and smoking as compared to the baseline group (Table 5).
| CYPIA1 | Smoking | Controls | Cases | OR1 | 95%CI | |
| A/A | None | 21 | 21 | 1.00 | ||
| Yes | 14 | 32 | 1.53 | 0.47 | 4.96 | |
| A/G | None | 18 | 12 | 0.62 | 0.20 | 1.89 |
| Yes | 6 | 15 | 0.92 | 0.24 | 3.53 | |
| G/G | None | 3 | 13 | 4.90 | 0.90 | 26.73 |
| Yes | 0 | 9 | 19.02 | 7.27 | 54.06 | |
As shown in Table 5, when compared to the combined GSTM1 +/+ or +/O genotype with no H pylori infection at a baseline of 1.0, the OR of H pylori infection alone was 4.19 (95%CI: 1.16-15.13). In combined GSTM1 O/O genotype and H pylori infection group, the OR was 12-fold higher than that in the baseline group (Table 6).
As shown in Table 7, when compared to the combined GSTM1 +/+ or +/O genotype with no smoking at a baseline of 1.0, the OR of smoking alone was 1.22 (95%CI: 0.37-4.01), and the OR of GSTM1 O/O alone was 3.08 (95%CI: 1.11-8.54). An interaction between the GSTM1 O/O genotype and smoking was also observed with a fourfold increase compared to that in the baseline group.
In this study, since the biopsy from cases and controls was tested by gastroscopy, there was no bias caused by misclassification. The results of this study showed that smoking and H pylori infection had an association with stomach cancer after being adjusted for age, sex, education, and occupation. Jarebinski et al[12] in 1992 first reported that there is a weak association between smoking and stomach cancer. Liu and Wang[13] in 2002 made a meta-analysis of the relationship between smoking and stomach cancer, and found that cigarette smoking is a risk factor for gastric cancer, especially for male smokers. Finding from most case-control and cohort studies support the causal relation between smoking and gastric cancer[14-16]. Epidemiological evidence relating H pylori infection to the etiology of gastric cancer showed that at least 30% of gastric cancers in the developing world and 50% of gastric cancers in the developed world may be attributed to H pylori infection[17-20]. Studies indicate that H pylori infection is a frequent finding in patients with gastric adenocarcinoma and benign peptic ulcer[21-23]. With respect to carcinogenic mechanism, H pylori affects several aspects of gastric epithelial cell function[24-26]. The result from the present study is in agreement with these reports.
There are a number of studies on cytochrome p450 and GSTs associated with cancers, such as breast, lung, liver, esophagus, and stomach cancer[6-8,11].
It was reported that in the presence of p450, CYPIA, CYP2E1, and CYP3A in stomach cancer, the expression of CYPIA (51%) and CYP3A (28%) increases in stomach cancer[27-29]. Liakhovich et al[30] reported that the frequency of CYPIA1-val allele in lung, stomach, and intestine cancer groups was three to five times higher than in the healthy control group. The carriers of homozygous GSTM1 gene deletion are also higher lung cancer risk.
Cai et al[31] in 1999 reported that GSTM1 null genotype correlates with the susceptibility to gastric cancer (OR = 2.03, 95%CI of OR = 1.09-3.80). The risk for gastric cancer in those with GSTM1 null and GSTT1 non-null genotype is significantly higher than that in those with GSTM1 non-null and GSTT1 null genotype, with an OR of 2.91 (95%CI, 1.09-7.89). The risk for gastric cancer in smokers with GSTM1 null genotype increases significantly (OR = 8.06, 95%CI, 2.83-23.67)[31].
Setiawan et al[32] reported that the prevalence of GSTM1 null genotype is 48% in gastric cancer cases, 60% in CG patients, and 51% in controls; after controlling for age, gender, education, pack-years of smoking, alcohol drinking, body mass index, H pylori infection, fruit and salt intake, the adjusted OR for GSTT1 and gastric cancer was 2.50 (95%CI, 1.01-6.22); when gastric cancer cases are compared to CG patients, the adjusted OR for GSTT1 is 2.33 (95%CI, 0.75-7.25).
In conclusion, CYPIA1 G/G and GSTM1 O/O genotypes are associated with the increase of stomach cancer in Chinese people. When the CYPIA1 G/G genotype for patients is combined with GSTM1 O/O genotype, then the risk factors involved are H pylori infection or smoking. A study needs to be carried out in future.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Agundez JA. Cytochrome P450 gene polymorphism and cancer. Curr Drug Metab. 2004;5:211-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1107] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 3. | You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, Li JY, Jin ML, Hu YR, Yang CS. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92:1607-1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Garte S. The role of ethnicity in cancer susceptibility gene polymorphisms: the example of CYP1A1. Carcinogenesis. 1998;19:1329-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Shi SF, Xie QX, LI XB. The advance process in relation to the susceptibility of tumor. Guowai Yixue Zhongliuxue Fence. 1997;4:304-307. [Cited in This Article: ] |
| 6. | Smith GB, Harper PA, Wong JM, Lam MS, Reid KR, Petsikas D, Massey TE. Human lung microsomal cytochrome P4501A1 (CYP1A1) activities: impact of smoking status and CYP1A1, aryl hydrocarbon receptor, and glutathione S-transferase M1 genetic polymorphisms. Cancer Epidemiol Biomarkers Prev. 2001;10:839-853. [PubMed] [Cited in This Article: ] |
| 7. | Taioli E, Ford J, Trachman J, Li Y, Demopoulos R, Garte S. Lung cancer risk and CYP1A1 genotype in African Americans. Carcinogenesis. 1998;19:813-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW. Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst. 1993;85:1159-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 467] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 9. | Crofts F, Taioli E, Trachman J, Cosma GN, Currie D, Toniolo P, Garte SJ. Functional significance of different human CYP1A1 genotypes. Carcinogenesis. 1994;15:2961-2963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 203] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Hayashi SI, Walanabe J, Nakachi K, Kawajiri K. PCR detection of an A/G polymorphism within exon 7 of the CYPIA1 gene. Nucleic Acids Res. 1991;19:4797. [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Shields PG, Ambrosone CB, Graham S, Bowman ED, Harrington AM, Gillenwater KA, Marshall JR, Vena JE, Laughlin R, Nemoto T. A cytochrome P4502E1 genetic polymorphism and tobacco smoking in breast cancer. Mol Carcinog. 1996;17:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 12. | Jarebinski M, Adanja B, Vlajinac H, Pekmezović T, Sipetić S. [Evaluation of the association of cancer of the esophagus, stomach and colon with habits of patients]. Vojnosanit Pregl. 1992;49:19-24. [PubMed] [Cited in This Article: ] |
| 13. | Liu YX, Wang JZ. [Meta-analysis of the relationship between smoking and stomach cancer]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002;24:559-563. [PubMed] [Cited in This Article: ] |
| 14. | González CA, Pera G, Agudo A, Palli D, Krogh V, Vineis P, Tumino R, Panico S, Berglund G, Simán H. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer. 2003;107:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V. Alcohol consumption, smoking and risk of gastric cancer: case-control study from Moscow, Russia. Cancer Causes Control. 2000;11:363-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Chow WH, Swanson CA, Lissowska J, Groves FD, Sobin LH, Nasierowska-Guttmejer A, Radziszewski J, Regula J, Hsing AW, Jagannatha S. Risk of stomach cancer in relation to consumption of cigarettes, alcohol, tea and coffee in Warsaw, Poland. Int J Cancer. 1999;81:871-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 17. | Forman D. Helicobacter pylori and gastric cancer. Scand J Gastroenterol Suppl. 1996;220:23-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Guisset M, Coton T, Rey P, Debonne JM. [Helicobacter pylori infection in developing countries]. Med Trop (Mars). 1997;57:77-82. [PubMed] [Cited in This Article: ] |
| 19. | Tokunaga Y, Hata K, Ryo J, Kitaoka A, Tokuka A, Ohsumi K. Density of Helicobacter pylori infection in patients with peptic ulcer perforation. J Am Coll Surg. 1998;186:659-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Lin CW, Chang YS, Wu SC, Cheng KS. Helicobacter pylori in gastric biopsies of Taiwanese patients with gastroduodenal diseases. Jpn J Med Sci Biol. 1998;51:13-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Basso D, Navaglia F, Brigato L, Piva MG, Toma A, Greco E, Di Mario F, Galeotti F, Roveroni G, Corsini A. Analysis of Helicobacter pylori vacA and cagA genotypes and serum antibody profile in benign and malignant gastroduodenal diseases. Gut. 1998;43:182-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Buckley MJ, O'Shea J, Grace A, English L, Keane C, Hourihan D, O'Morain CA. A community-based study of the epidemiology of Helicobacter pylori infection and associated asymptomatic gastroduodenal pathology. Eur J Gastroenterol Hepatol. 1998;10:375-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Röher HD, Verreet PR, Wörmer O, Müller FP, Ohmann C, Fischbach W. Helicobacter pylori in the upper gastrointestinal tract: medical or surgical treatment of gastric lymphoma? Langenbecks Arch Surg. 2000;385:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Törgyekes E, Molnár B. [Effect of Helicobacter pylori infection on the apoptotic and cell proliferative processes of the gastric mucosa]. Orv Hetil. 1999;140:755-760. [PubMed] [Cited in This Article: ] |
| 25. | Calam J. Host mechanisms: are they the key to the various clinical outcomes of Helicobacter pylori infection? Ital J Gastroenterol Hepatol. 1997;29:375-382. [PubMed] [Cited in This Article: ] |
| 26. | De Gusmão VR, Nogueira Mendes E, De Magalhães Queiroz DM, Aguiar Rocha G, Camargos Rocha AM, Ramadan Ashour AA, Teles Carvalho AS. vacA genotypes in Helicobacter pylori strains isolated from children with and without duodenal ulcer in Brazil. J Clin Microbiol. 2000;38:2853-2857. [PubMed] [Cited in This Article: ] |
| 27. | Suzuki S, Muroishi Y, Nakanishi I, Oda Y. Relationship between genetic polymorphisms of drug-metabolizing enzymes (CYP1A1, CYP2E1, GSTM1, and NAT2), drinking habits, histological subtypes, and p53 gene point mutations in Japanese patients with gastric cancer. J Gastroenterol. 2004;39:220-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Hamajima N. Genetic polymorphisms and cancer risk. Gan To Kagaku Ryoho. 2004;31:853-857. [PubMed] [Cited in This Article: ] |
| 29. | Murray GI, Taylor MC, Burke MD, Melvin WT. Enhanced expression of cytochrome P450 in stomach cancer. Br J Cancer. 1998;77:1040-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Liakhovich VV, Vavilin VA, Gutkina NI, Laktionova IP, Makarova SI, Mitrofanov DV, Ostashevskiĭ VA, Chasovnikova OB. Genes and enzymes of the xenobiotic-metabolizing system in cancer pathology. Vopr Med Khim. 1997;43:330-338. [PubMed] [Cited in This Article: ] |
| 31. | Cai L, Yu S, Chen J. Relationship between glutathione S-transferase M1, T1 genotype and susceptibility to gastric cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 1999;33:331-333. [PubMed] [Cited in This Article: ] |
| 32. | Setiawan VW, Zhang ZF, Yu GP, Li YL, Lu ML, Tsai CJ, Cordova D, Wang MR, Guo CH, Yu SZ. GSTT1 and GSTM1 null genotypes and the risk of gastric cancer: a case-control study in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2000;9:73-80. [PubMed] [Cited in This Article: ] |