Published online Aug 14, 2005. doi: 10.3748/wjg.v11.i30.4667
Revised: December 23, 2004
Accepted: December 26, 2004
Published online: August 14, 2005
AIM: Cell adhesion molecules and their signal molecules play a very important role in carcinogenesis. The aim of this study is to elucidate the role of these molecules and the signal molecules of integrins and E-cadherins, such as (focal adhesion kinase) FAK, (integrin linked kinase) ILK, and β-catenin in hepatocellular carcinoma cell apoptosis.
METHODS: We first synthesized the small molecular compound, S-(1,2-dichlorovinyl)-L-cysteine (DCVC), and identified it, by element analysis and 1H NMR. To establish the apoptosis model of the SMMC-7721 hepatocellular carcinoma cell, we treated cells with DCVC in EBSS for different concentrations or for various length times in the presence of 20 μmol/L N,N’-diphenyl-p-phenylenediamine, which blocks necrotic cell death and identified this model by flow cytometry and DNA ladder. Then we studied the changes of FAK, ILK, β-catenin, and PKB in this apoptotic model by Western blot.
RESULTS: We found that the loss or decrease of cell adhesion signal molecules is an important reason in apoptosis of SMMC-7721 hepatocellular carcinoma cell and the apoptosis of SMMC-7721 cell was preceded by the loss or decrease of FAK, ILK, PKB, and β-catenin or the damage of cell-matrix and cell-cell adhesion.
CONCLUSION: Our results suggested that the decrease of adhesion signal molecules, FAK, ILK, PKB, and β-catenin, could induce hepatocellular carcinoma cell apoptosis.
- Citation: Su JM, Wang LY, Liang YL, Zha XL. Role of cell adhesion signal molecules in hepatocellular carcinoma cell apoptosis. World J Gastroenterol 2005; 11(30): 4667-4673
- URL: https://www.wjgnet.com/1007-9327/full/v11/i30/4667.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i30.4667
Apoptosis or programmed cell death is critical for normal development and tissue homeostasis[1]. However uncontrolled apoptosis may occur after treatment with cytostatic chemicals. It is a pathophysiological process and is associated with the occurrence of various human diseases[2]. Maintenance of cell-matrix or cell-cell contact is an important cell survival factor[3-6] and loss of these contacts, or rounding up, is a hallmark of apoptosis. Cell-matrix interactions occur at the closest contact between the cell and the substratum. It was called as focal adhesion. Integrins are mainly engaged in cell-matrix adhesion. Integrin ligated with the extracellular matrix results in activation of focal adhesion kinase (FAK) and integrin linked kinase (ILK). FAK is a 125-ku protein tyrosine kinase, that is critical in transmission of signals from the focal adhesion to the cytoplasm after cell attachment[7,8]. ILK is another integrin cytoplasmic-binding protein that has been implicated in the regulation of cell adhesion and extracellular matrix deposition as well as the activation of cell survival and proliferative pathways, including those involving MAP kinase, PKB/Akt and GSK-3β[9,10]. Similarly, E-cadherin is a calcium-dependent transmembrane cell-cell adhesion protein, mainly involved in cell-cell adhesion[11]. When the homophilic interaction in their specific extra-cellular regions occurred, their intra-cellular linked protein, β-catenin, was activated and mediated signal transduction[12,13]. It seems clear that FAK, ILK, and β-catenin are important for signaling, cell attachment and cell survival. Despite the importance of these proteins in signal transduction and cell survival, there is little evidence about the role of FAK, ILK, and β-catenin in chemically induced models of apoptotic cell death. Therefore, we have investigated the role of FAK, ILK, β-catenin and other related signal molecule such as PKB in SMMC-7721 hepatocellular carcinoma cell using the characterized nephrotoxicant, S-(1,2-dichlorovinyl)-L-cysteine (DCVC)[14,15]. DCVC is metabolized by a β-lyase to a reactive acylating metabolite that covalently modifies cellular macromolecules[16-18]. The apoptosis of renal proximal tubule epithelial cell (RPTE) induced by DCVC had been reported. Cell death of primary cultured RPTE caused by DCVC treatment is preceded by disorganization of the cytoskeletal network and dissolution of focal adhesion[14,15]. Thus, this compound is a useful agent to study the role of cell-cell or cell-matrix adhesion in apoptosis of epithelial cells. To study the relationship between the cell adhesion signal molecules and cell apoptosis, we found the apoptosis model of the SMMC-7721 hepatocellular carcinoma cell using DCVC and examined the role of FAK, ILK, β-catenin and other related signal molecules in this model.
SMMC-7721 cells were obtained from the Department of Pathology, Shanghai No. 2, Military Medical University (Shanghai, China)[19]. Anti-FAK and anti-ILK polyclonal antibodies were purchased from Santa Cruz. Anti-β-catenin and anti-PKB mAb were purchased from Sigma. Secondary antibodies conjugated with HRP were purchased from Watson Biotech. (Shanghai). N,N’-Diphenyl-p-phenylenediamine (DPPD) was from Sigma-Aldrich (St. Louis, MO, USA). DCVC was synthesized by us.
SMMC-7721 cells were grown in RPMI medium 1640 supplemented with penicillin and 10% heat inactivated fetal bovine serum in 37°C and 50 mL/L CO2. Confluent monol-ayers of SMMC-7721 in 10-cm dishes were washed with Earle’s balanced salt solution (EBSS) twice. Thereafter, cells were treated with DCVC in EBSS for different concentrations or for various lengths of time. To find the apoptosis model, cells were treated with DCVC in the presence of 20 µmol/L DPPD (stocked in dimethyl sulfoxide), which blocks necrotic cell death but allows the onset of apoptosis[15,20]. Following treatment with DCVC, cells were allowed to recover in a complete medium containing 20 µmol/L DPPD.
After treatment of DCVC and recovery in complete medium, confluent cells were washed twice with ice-cold PBS and lysed in modified loading buffer containing 50 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol and protease inhibitors (1 mmol/L PMSF). The samples were boiled for 10 min and centrifuged at 12 000 g for 10 min, and insoluble material was removed. Equal amount of protein were separated on a SDS-PAGE and transferred to PVDF membrane. After being blocked with 3% BSA in PBS (containing 0.05% Tween 20), the membrane was incubated with the appropriate primary antibodies, followed by HRP-conjugated secondary antibodies. Proteins were visualized by fluorography using an enhanced chemiluminescence system (Perfect Biotech, Shanghai).
Dry L-cysteine (0.1 mol) and sodium were added in portions to 300 mL of dry ammonia contained in a flask equipped with a magnetic stirrer and a drying tube. The reaction flask was set in a dish, so that alcohol could be applied to prevent frosting. Sodium was added first to give the characteristic blue color, which disappeared when cysteine was added to form the disodium salt. Trichloroethylene (8.96 mL, 0.1 mole) was dissolved slowly in liquid ammonia (25 mL). After 30 min, a stream of air was directed on the reaction flask to speed up evaporation of ammonia, which required about 2 h. The slightly colored residue was dissolved in 300 mL of water, free from traces of ammonia in vacuo and, by the addition of glacial acetic acid, adjusted to pH 5.0 from an initial pH 11.9. The resulting copious precipitate was diluted with one volume of ethanol, cooled overnight in a refrigerator and isolated by filtration. The crude precipitate was dissolved in 600 mL of water at 70°C, 1 g of activated carbon was added, and the mixture was filtered while it was still hot. An equal volume of ethanol was added to the clear filtrate and, after overnight at 4°C, 9.1 g of needle-like crystals were obtained. MP 158-159°C.
DCVC analysis, calculated for C5H7NO2SCl2: C, 27.79; H, 3.27; N, 6.48; S, 14.84; Cl, 32.82. Found: C, 27.82; H, 3.28; N, 6.47; S, 14.94; Cl, 32.80. 1H NMR (deuterium replaced DMSO as solvent 300 mol/L) δ: 3.35 (t, J = 15.50 Hz, J = 7.42 Hz, 1 H), 3.53 (t, J = 15.50 Hz, J = 4.05 Hz, 1 H), 3.91 (t, J = 4.05 Hz, J = 7.42 Hz, 1 H), 6.71 (s, 1 H). FAB MS: 217 ([MH+]).
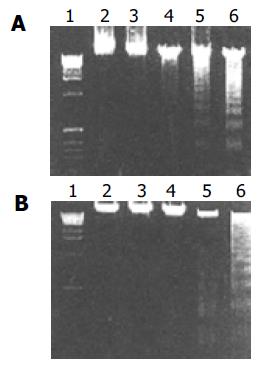
DNA laddering was determined by agarose gel electrophoresis. Cells were harvested by scraping the adherent cells which were then combined with floating cells present in the culture medium. After centrifugation, the cell pellet was washed once with ice-cold PBS by centrifugation, lysed with lysis buffer (10 mmol/L Tris, 1 mmol/L EDTA, and 2 g/L Triton X-100, pH 7.4), and incubated on ice for 20 min. Cell debris was removed by centrifugation; the supernatant was treated with RNase (60 µg/mL) for 1 h at 50°C. The DNA was precipitated with 0.5 mol/L NaCl and equal volume of isopropyl alcohol and separated by electrophoresis on 1% agarose gels.
SMMC-7721 hepatocellular carcinoma cell nuclear fragmentation treated with DCVC was stained with 1 µg/mL Hoechst 33258 in PBS. After washing with PBS, coverslips were mounted and viewed using a Nikon epifluorescence microscope.
Apoptosis was also determined by cell cycle analysis. Both floating and adherent cells that were trypsinized were pooled and fixed in 100% ethanol (-20°C). After washing the cells twice with PBS, cells were resuspended in PBS containing 10 µg/mL RNase A and 7.5 µmol/L propidium iodide. After 30 min incubation at room temperature, the cell cycle was analyzed by flow cytometry (FAC Scan, Becton Dickinson) and the percentage of cells present in sub-G0/G1 was calculated using the LYSIS software (Becton Dickinson).
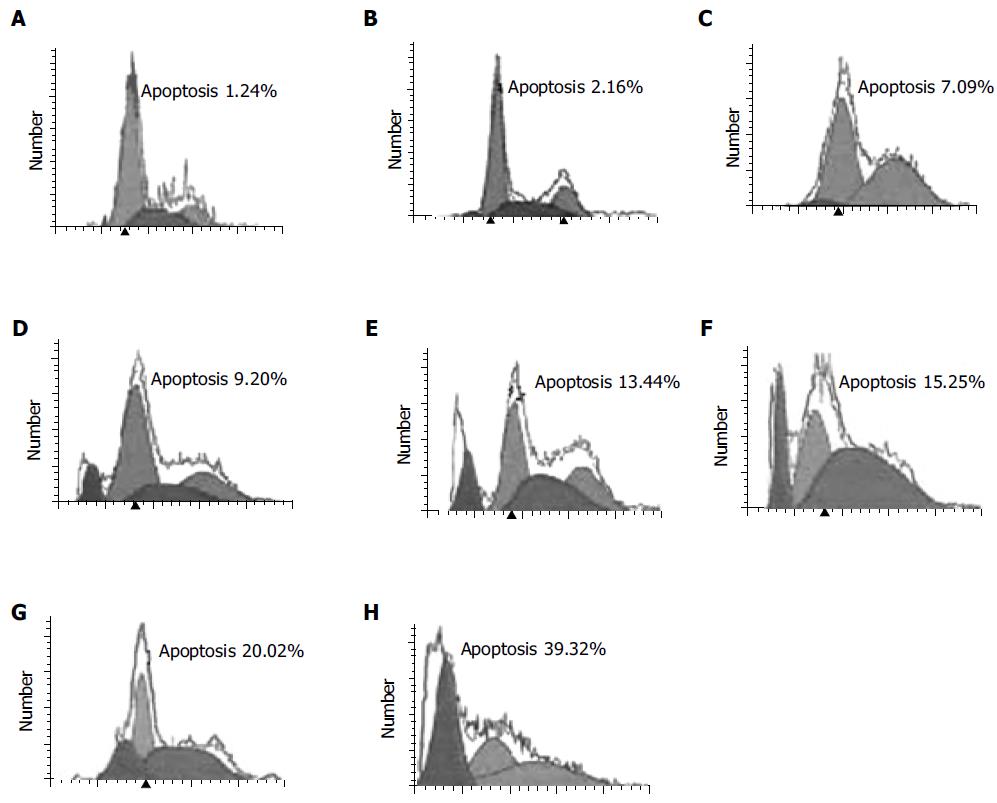
SMMC-7721 hepatocellular carcinoma cells were induced into apoptosis in the presence of DCVC (Figure 1). When SMMC-7721 hepatocellular carcinoma cells were treated with 0.02 mmol/L DPPD and various DCVC concentrations for 6 h and then harvested for flow cytometry assay, it was found that the cell apoptosis was induced by DCVC in a dose-dependent manner in the presence of DPPD and when the concentration of DCVC is larger than 0.05 mmol/L, the percentage of cell apoptosis increased rapidly (Figure 2).
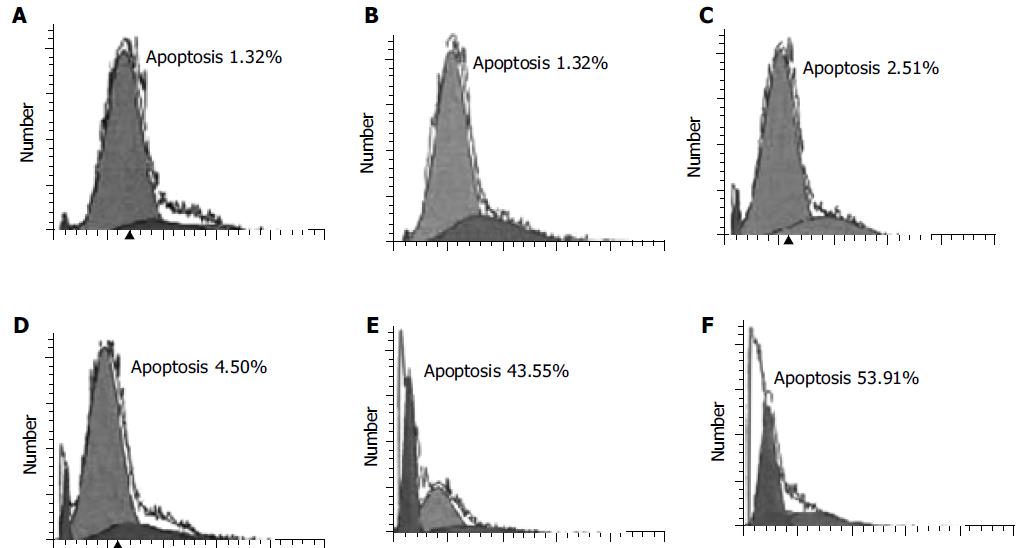
Similarly, the effect of DCVC on apoptosis was also time-dependent. At the first 4 h, the percentage of cell apoptosis was very low and after that it increased rapidly (Figure 3).
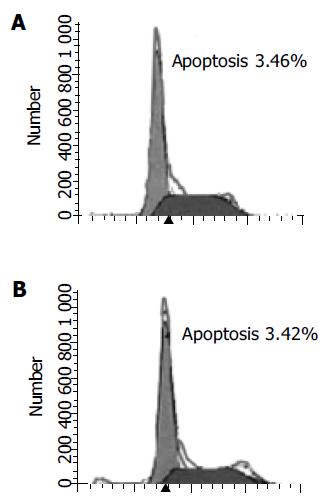
These data demonstrate that the effective apoptosis had not happened until the DCVC concentration and treatment time was high and long enough. We also demonstrate that DPPD alone could not cause cell apoptosis (Figure 4).
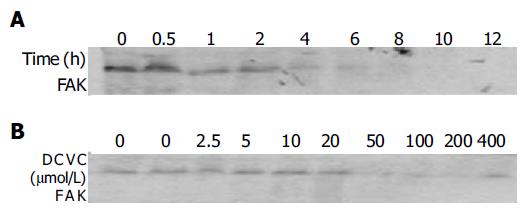
FAK, as an important cell survival factor, has been implicated in apoptosis induced by various stimuli[21-23]. Until now, no reports have described the role of FAK in DCVC/DPPD-induced malignant cell apoptosis. We investigated the FAK expression in DCVC-induced SMMC-7721 hepatocellular carcinoma cell by Western blot analysis and found that the level of FAK protein was almost lost (Figure 5).
Interestingly, we found that the FAK expression decreased in a dose-dependent and time-dependent manner. When the cells were treated for 1 h, the protein level of FAK began to decrease, but at that time the percentage of cell apoptosis had no significant decrease. After the treatment time gets longer than 4 h, the FAK protein was almost lost (Figure 5A) and the percentage of cell apoptosis began to increase rapidly. When the concentration of DCVC was larger than 0.05 mmol/L, the FAK expression almost was not observed (Figure 5B). These results were consistent with the process of cell apoptosis and suggested that cell apoptosis occurred after the cleavage of FAK and the damage of cell-matrix interaction or the focal adhesion.
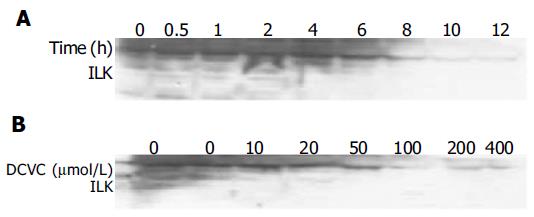
ILK is another newly identified, integrin cytoplasmic-binding protein. Overexpression of ILK in cultured intestinal and mammary epithelial cells has been previously shown to induce changes characteristic of oncogenic transformation, including anchorage-independent growth, invasiveness, suppression of anoikis and tumorigenicity in nude mice[9]. For this reason, we measured the expression of ILK in DCVC-induced SMMC-7721 hepatocellular carcinoma cell by Western blot analysis and found that the level of ILK was also decreased (Figure 6). These findings further demonstrated that the disruption of focal adhesion was taking place in this apoptosis model.
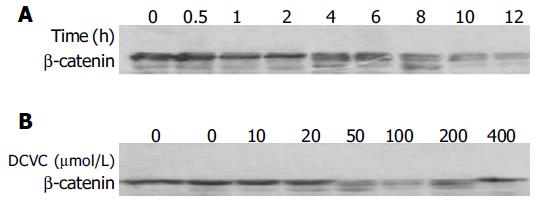
E-cadherin-mediated cell-cell adhesion plays a crucial role in intercellular communication, which is related to the regulation of cell proliferation, differentiation, and apoptosis[24]. β-Catenin regulates cadherin-mediated cell-cell adhesion and also functions as a signaling molecule[25]. For this reason, we measured the expression of β-catenin in DCVC-induced SMMC-7721 hepatocellular carcinoma cell by Western blot analysis and interestingly found that the level of β-catenin began to decrease for an hour of DCVC treatment and after that, the level of β-catenin decreased further (Figure 7A). Similarly when the dose of DCVC was larger than 0.05 mmol/L, β-catenin also decreased (Figure 7B). These findings suggest that, the cell apoptosis occurred after the decrease of β-catenin and the damage of intercellular adhesion.
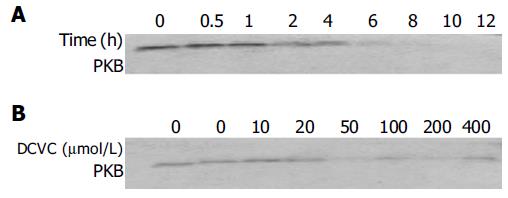
PKB has been demonstrated to play a suppressive role in cell apoptosis[26]. We therefore investigated the PKB level in response to DCVC in the presence of DPPD. We found when the time of DCVC treatment was longer or the dose of DCVC was increased, the level of PKB decreased. Particularly, after 4 h of DCVC treatment, PKB obviously decreased and after 8 h, PKB protein almost disappeared (Figure 8).
In this study, we investigated the level of FAK, ILK, PKB, and β-catenin during apoptosis of SMMC-7721 hepatocellular carcinoma cell induced by DCVC in the presence of DPPD. It was found that the level of each protein mentioned above dramatically decreased. These results suggest that the loss or decrease of cell adhesion signal molecules is an important reason in apoptosis of SMMC-7721 hepatocellular carcinoma cell. The apoptosis of SMMC-7721 was preceded by the loss or decrease of FAK, ILK, PKB, and β-catenin or the damage of cell-matrix and cell-cell adhesion.
FAK has been shown to play a suppressive role in apoptosis induced by various stimuli including H2O2 and c-myc, and apoptosis induced by keeping cell in suspension, termed anoikis, which disrupts the interactions between normal epithelial cells and the extracellular matrix[21,27,28]. Our findings strongly support the view that FAK play a suppressive role in apoptosis. Furthermore, decrease of FAK seems to precede cell apoptosis.
ILK is another integrin cytoplasmic-binding protein that has been identified as a potential PDK-2. ILK is capable of phosphorylating PKB/Akt on Ser-473 and stimulating its activity. Attwell found that overexpression of ILK in SCP2 mouse mammary epithelial cells results in a profound inhibition of anoikis[29]. Until the present day, no reports have described the role of ILK in the apoptosis of human hepatocellular carcinoma cell. We found that the level of ILK was decreased in DCVC/DPPD-induced apoptosis. This finding is in accordance with that of Attwell’s reports[29].
β-catenin has emerged as an important oncogene in the transformation of a number of epithelial cancers[30]. It acts as a downstream transcriptional activator of the Wingless-Wnt signaling pathway. The β-catenin-Tcf complex transactivates the downstream genes that regulate cell proliferation or inhibit apoptosis[31]. But Kim et al[32] reported that overexpressed β-catenin induces apoptosis in NIH 3T3 fibroblasts, corneal fibroblasts, corneal epithelia, uveal melanoma cells, and several carcinoma cell lines. In this study, we found that the level of β-catenin was dramatically decreased in DCVC-induced SMMC-7721 cell apoptosis and the reason needs to be elucidated by further research.
PKB acted as a common downstream signal protein of FAK, and ILK has been demonstrated to play a suppressive role in cell apoptosis[21,26,29]. Our results showed that the PKB level was decreased with increased DCVC concentration or treatment time, as well as the percentage of apoptotic cells during the apoptosis induced by DCVC in the presence of DPPD.
Our findings indicate that DCVC cause the cleavage of FAK, ILK, β-catenin, and PKB. Cleavage of these proteins may actually ensure that the cell does not attach to matrix or other cells. It blocks the cell-matrix and cell-cell adhesion-mediated signal transduction and gives rise to cell apoptosis. It demonstrates the importance of cell adhesion signal molecules in cell survival. However, the exact way of cleavage of these proteins is still unclear. FAK and β-catenin may be cleavaged by caspases, as both have been identified as substrates for caspases[33,34]. Whether ILK and PKB are also cleaved by caspases or other enzymes, remains to be determined.
In conclusion, the present findings demonstrate that the cell apoptosis occurred after the damage of cell-matrix interaction and cell-cell contact. Cell-matrix and cell-cell adhesionmediated signal transduction is fundamental for cell survival and many cell adhesion signal molecules such as FAK, ILK, and β-catenin have oncogenic potential and have anti-apoptotic function.
We gratefully appreciate Dr. Hong-Xing Zhang for the assistance of DCVC synthesis. We thank all people in the Flow Cytometry Core Facility of Tumor Hospital at Fudan University for processing the cell apoptosis analysis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Sanders EJ, Wride MA. Programmed cell death in development. Int Rev Cytol. 1995;163:105-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Osborne BA. Induction of genes during apoptosis: examples from the immune system. Semin Cancer Biol. 1995;6:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Zachary I, Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992;71:891-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 381] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 256] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 380] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161-6165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 451] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 7. | Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439-23442. [PubMed] [Cited in This Article: ] |
| 8. | Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192-5196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1028] [Cited by in F6Publishing: 1124] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 9. | White DE, Cardiff RD, Dedhar S, Muller WJ. Mammary epithelial-specific expression of the integrin-linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene. 2001;20:7064-7072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Guo L, Sanders PW, Woods A, Wu C. The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am J Pathol. 2001;159:1735-1742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Nose A, Nagafuchi A, Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO J. 1987;6:3655-3661. [PubMed] [Cited in This Article: ] |
| 12. | Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 582] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 13. | Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr Biol. 1997;7:308-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 302] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Van de Water B, Jaspers JJ, Maasdam DH, Mulder GJ, Nagelkerke JF. In vivo and in vitro detachment of proximal tubular cells and F-actin damage: consequences for renal function. Am J Physiol. 1994;267:F888-F899. [PubMed] [Cited in This Article: ] |
| 15. | Van de Water B, Kruidering M, Nagelkerke JF. F-actin disorganization in apoptotic cell death of cultured rat renal proximal tubular cells. Am J Physiol. 1996;270:F593-F603. [PubMed] [Cited in This Article: ] |
| 16. | Stevens JL, Robbins JD, Byrd RA. A purified cysteine conjugate beta-lyase from rat kidney cytosol. Requirement for an alpha-keto acid or an amino acid oxidase for activity and identity with soluble glutamine transaminase K. J Biol Chem. 1986;261:15529-15537. [PubMed] [Cited in This Article: ] |
| 17. | Hayden PJ, Stevens JL. Cysteine conjugate toxicity, metabolism, and binding to macromolecules in isolated rat kidney mitochondria. Mol Pharmacol. 1990;37:468-476. [PubMed] [Cited in This Article: ] |
| 18. | Chen Q, Jones TW, Brown PC, Stevens JL. The mechanism of cysteine conjugate cytotoxicity in renal epithelial cells. Covalent binding leads to thiol depletion and lipid peroxidation. J Biol Chem. 1990;265:21603-21611. [PubMed] [Cited in This Article: ] |
| 19. | Cai T, Lei QY, Wang LY, Zha XL. TGF-beta 1 modulated the expression of alpha 5 beta 1 integrin and integrin-mediated signaling in human hepatocarcinoma cells. Biochem Biophys Res Commun. 2000;274:519-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Zhan Y, Cleveland JL, Stevens JL. A role for c-myc in chemically induced renal-cell death. Mol Cell Biol. 1997;17:6755-6764. [PubMed] [Cited in This Article: ] |
| 21. | Sonoda Y, Watanabe S, Matsumoto Y, Aizu-Yokota E, Kasahara T. FAK is the upstream signal protein of the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen peroxide-induced apoptosis of a human glioblastoma cell line. J Biol Chem. 1999;274:10566-10570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 202] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Xu LH, Owens LV, Sturge GC, Yang X, Liu ET, Craven RJ, Cance WG. Attenuation of the expression of the focal adhesion kinase induces apoptosis in tumor cells. Cell Growth Differ. 1996;7:413-418. [PubMed] [Cited in This Article: ] |
| 23. | Crouch DH, Fincham VJ, Frame MC. Targeted proteolysis of the focal adhesion kinase pp125 FAK during c-MYC-induced apoptosis is suppressed by integrin signalling. Oncogene. 1996;12:2689-2696. [PubMed] [Cited in This Article: ] |
| 24. | Yang SZ, Kohno N, Kondo K, Yokoyama A, Hamada H, Hiwada K, Miyake M. Adriamycin activates E-cadherin-mediated cell-cell adhesion in human breast cancer cells. Int J Oncol. 1999;15:1109-1115. [PubMed] [Cited in This Article: ] |
| 25. | Ninomiya I, Endo Y, Fushida S, Sasagawa T, Miyashita T, Fujimura T, Nishimura G, Tani T, Hashimoto T, Yagi M. Alteration of beta-catenin expression in esophageal squamous-cell carcinoma. Int J Cancer. 2000;85:757-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 26. | Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1734] [Cited by in F6Publishing: 1705] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 27. | Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 830] [Cited by in F6Publishing: 828] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 28. | Ilić D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 376] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811-3815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Wright K, Wilson P, Morland S, Campbell I, Walsh M, Hurst T, Ward B, Cummings M, Chenevix-Trench G. beta-catenin mutation and expression analysis in ovarian cancer: exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int J Cancer. 1999;82:625-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 31. | Kim YS, Kang YK, Kim JB, Han SA, Kim KI, Paik SR. beta-catenin expression and mutational analysis in renal cell carcinomas. Pathol Int. 2000;50:725-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Kim K, Pang KM, Evans M, Hay ED. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell. 2000;11:3509-3523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056-26061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 259] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Brancolini C, Lazarevic D, Rodriguez J, Schneider C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J Cell Biol. 1997;139:759-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |