INTRODUCTION
Transcatheter arterial chemoembolization (TACE) has become the standard treatment for patients with unresectable hepatocellular carcinoma (HCC). The goal of TACE is to cause tumor necrosis and control tumor growth while preserving as much functional liver tissue as possible. The ultimate purpose is to prolong life. However, TACE’s therapeutic effects also have some limits and the combination of TACE with other therapies such as percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), irradiation, etc., are superior to single TACE for improving the prognosis and survival of patients with HCC[1]. We reported a case of recurrent HCC and the successful management with combination of p53 gene therapy and TACE.
CASE REPORT
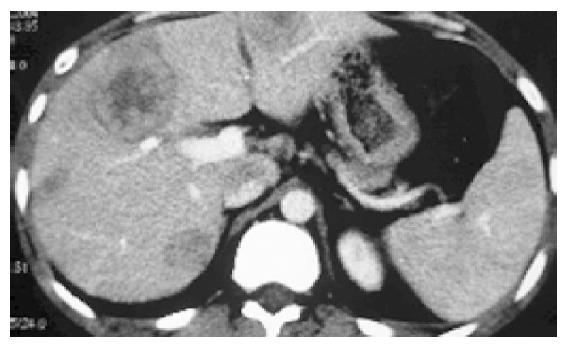
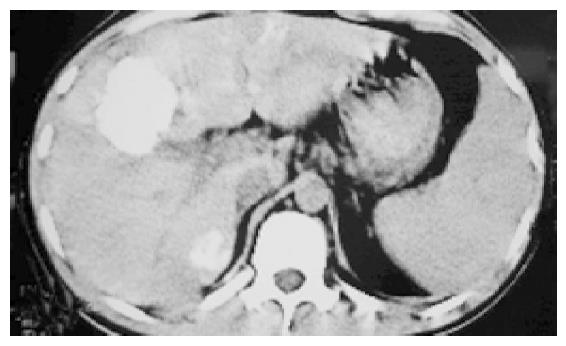
A 23-year old man with HCC in right lobe was treated with right lobe irregular hepatectomy 20 mo ago. Five months after the procedure, several recurrent nodules were found in the remnant liver in a routine post-operation CT scan. This patient did not have any clinical symptom. Afterward, the patient received traditional Chinese medicine therapy, but no effect was found. And some clinical manifestations of liver function impairment such as belly swollen, lose of appetite and jaundice occurred gradually with AFP (alpha-fetoprotein)>1210 ng/mL. Three months ago, the patient presented at the hospital. In the light of the multiple hepatic nodules (Figure 1), which were not indicated for re-operation, we were determined to treat this patient with p53 gene therapy combining TACE. Firstly, we punctured the largest nodule with fine needle percutaneously under the guidance of CT and after the tip of needle was confirmed within the largest nodule, p53 (Gendicine, Shenzhen Sibiono Bentech, China) was injected into intra-tumor in a fashion of multiple injections. Then, we infused p53 through hepatic artery transcatheterally in our catheter room. A total of 3×1012 VP (virus particles) were administered. After the procedure, the patient had a moderate fever ranging 38-38.5 °C and no complication was observed. Four days later, we super-selectively embolized the patient’s hepatic arteries with 5-Fu, vinorelbine, iodized oil. After the uneventful post-operative 30 d, the CT examination of the abdomen was made and the image demonstrated the complete deposit of oil and no recurrence sign was identified (Figure 2). In the following 2 mo (until this paper was written), the patient was in good clinical condition with normal liver function and no recurrence was identified.
Figure 1 The CT image of post-operation that revealed multiple hypodense nodules with circle contrast manifestation.
Figure 2 The CT image of post TACE d 30 that revealed recurrent nodules with homogeneous complete lipiodol uptake.
DISCUSSION
Hepatocellular carcinoma (HCC) is a highly malignant tumor with a very high morbidity and mortality. It has a poor prognosis due to its rapid infiltrating growth and complicating liver cirrhosis. Since TACE was introduced as a palliative treatment in patients with unresectable HCC, it has become one of the most common forms of interventional therapy[2-4]. However, its therapeutic effect is also limited by the lack of appropriate and reliable embolic agents and when the tumor is infiltrative in nature or is hypovascular, too large or too small[5]. Another limitation of TACE is the need for repeated treatments, which can result in deterioration of liver function[6]. The combination of limited TACE with PEI or RFA, irradiation therapy may lead to improved survival and decreased risk of liver failure[1]. In this case, we adopted the combined therapy of p53 with TACE so as to overcome the downside of TACE.
p53 tumor suppressor gene is praised as gene guardian and the loss of p53 is thought to be responsible for the lack of apoptotic signals in tumor cells and thus for their uncontrolled proliferation and recurrence[7]. Many human tumors carry mutations in the p53[8,9] and mutant or absent p53 status has been associated with resistance to radiation therapy and to apoptosis-inducing chemotherapy[10]. The combination of p53 gene transduction with radiation or chemotherapy has resulted in local tumor control that is superior to either therapy alone[11,12]. In HCC, the incidence of p53 mutation was reported as 61%(17/28)[9]. Chen et al[13], also reported that mutations in the p53 gene were frequently detected in recurrent HCC and the interval between surgical resection and the recurrence of HCC was significantly longer in patients with the wild-type p53 gene than those with mutations, strongly suggesting a pathological role for the mutant p53 gene in HCC. Jeng et al[14], maintained that the biological behavior of the mutant p53 gene is strongly related to the invasiveness of HCC and may also influence the postoperative course. Many scholars suggest that the immunopositivity of the mutant p53 gene has a predictive role in the prognosis of patients with resected hepatocellular carcinoma[14].
Gendicine, recombinant human ad-p53 injection, (Shenzhen Sibiono Bentech, China) obtained a drug license from the state food and drug administration of China (SFDA; Beijing, China) and became the world’s first commercially-licensed gene therapy drug. Gendicine consists of advenovirus vectors and normal p53 tumor suppressor gene. Gendicine was used in clinical trials on patients with late-stage HNSCC (head and neck squamous cell carcinoma). After 8 wk of therapy involving one injection per week, 64% of patients’ tumors experienced complete regression and 32% experienced partial regression. In combination with chemo- and radiotherapy, it improved treatment efficacy more than three-fold.
Although the recommended indications are limited in HNSCC according to its specification, in this case, Gendicine was tried in the patient with recurrent HCC. Because of the multiple tumor nodules in liver parenchyma, this patient was not suitable for re-operation. So we tried the new gene drug. Three hours after the intra-tumor injection and transcatheter hepatic artery infusion with a total of 3×1012 VP (virus particles), Gendicine began to express P53 protein in tumor cell and reached a peak at post injection d 3. The P53 protein brings about specific anti-tumor cells effect in such ways as induction of apoptosis or necrosis, incentive of body immune response, regulation of cell cycle etc. We treated the patient with TACE at post injection d 4 in expectation of achieving an optimal therapeutic effect. Thirty days later, we used CT to evaluate the therapeutic effect. The CT scan images identified complete iodized oil uptake in tumor areas. HCC images that revealed a dense retention of lipiodol within the whole tumor or revealed no enhancement on contrast enchanced CT had a significantly higher necrotic rate. This patient’s images represent a significant tumor necrosis and imply a good prognosis in the long run. So, the patient manifested good clinical condition with normal liver function and no malignancy was identified in the next 2 mo.
This case demonstrated that the combination of p53 with TACE could be used in patients with HCC as a promising method and good efficacy could be gained.