Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2634
Revised: September 24, 2004
Accepted: November 4, 2004
Published online: May 7, 2005
AIM: To investigate the effect of interferon-α (IFN-α) on preventing or reversing hepatic fibrosis in rat experimental model induced by CCl4.
METHODS: One hundred and ten Sprague-Dawley rats were divided into five groups: group A (normal controls, n = 18), group B (fibrotic model controls, n = 22), group C (IFN-α prevention, n = 22) initially treated with intra-muscular injection of IFN-α in saline daily at the doses of 1×105 U for 6 wk, group D (IFN-α treatment, n = 24) treated with intra-muscular injection of IFN-α in saline daily at the doses of 1×105 U for 6 wk after the first 6 wk, group E (0.9% sodium chloride treatment control, n = 24) treated with intra-muscular injection of 0.01 mL/kg daily for 6 wk after the first 6 wk. At the end of the experiment, all rats of each group were killed. Samples of the liver obtained by biopsy were subjected to histological, immunohistochemical and electron microscopic studies for the expressions of transforming growth factor-β1 (TGF- β1) and α-smooth muscle actin (α-SMA).
RESULTS: The expressions of TGF-β1, the number of activated hepatic stellate cells and α-SMA in hepatic tissue of group C were significantly less than those of group B (P<0.01). The degree of fibrosis score in group B was also significantly less than that of group C under light microscope (P<0.01).
CONCLUSION: IFN-α can inhibit the production of TGF-β1, decrease HSC activation and stimulate its apoptosis.
- Citation: Chang XM, Chang Y, Jia A. Effects of interferon-alpha on expression of hepatic stellate cell and transforming growth factor-β1 and α-smooth muscle actin in rats with hepatic fibrosis. World J Gastroenterol 2005; 11(17): 2634-2636
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2634.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2634
Interferon-α (IFN-α) is a multi-functional cytokine which can prevent and treat hepatic fibrosis. Up to now, only a few studies on its antifibrotic mechanism are available[1-3]. The aim of this study was to investigate the antifibrotic mechanism of IFN-α by observing its effects on the expression of TGF-β1 and α-SMA in hepatic tissue.
Male Sprague-Dawley rats weighing 300±20 g were purchased from B-K Company of Shanghai. IFN-α was provided by Roche Pharmaceutic Limited Company of Shanghai. Trans-forming growth factor-β1 (TGF-β1), α-smooth muscle actin (α-SMA) were purchased from Boster Biological Engineering Limited Company.
One hundred and ten SD rats with free access to food and water for 1 wk before experiments were divided into five groups. Group A (normal controls, n = 18) was injected with 0.3 mL/100 g peanut oil subcutaneously and 0.01 mL/kg 0.9% NaCl per day for about 6 wk. Group B (fibrotic model controls, n = 22) was fed with a modified high fat diet containing 5 g/kg cholesterol, 200 g/kg lard and 1000 g/kg alcohol were injected subcutaneously with 400 mL/L CCl4 in peanut oil at a dose of 3 mL/kg twice a wk and the first dosage was 5 mL/kg. Group C (IFN-α preventing group, n = 22) was initially treated with intra-muscular injection of IFN-α in saline daily at the dose of 1×105 U for 6 wk. Group D (INF-α treatment group, n = 24) was treated with intra-muscular injection of interferon in saline daily at the dose of 1×105 U for 6 wk after the first 6 wk. Group E (0.9% sodium chloride treatment controls, n = 24) was administered daily with 0.9% sodium chloride daily for 6 wk after the first 6 wk. Six rats were randomly selected from groups A, B and C at the end of the 3rd and 6th wk and from groups D and E at the end of the 9th and 12th wk. The rats were killed and sections of the right lobes of the livers were fixed in 10 g/L formaldehyde solution, embedded in paraffin and then processed for light microscopy. Other tissue slices were fixed in 25 g/L glutaraldehyde for electron microscopy.
The right lobule livers were fixed with glutaraldehyde and ultrathin sections were stained with uranyl acetate and lead citrate. The ultrastructures of hepatocyte, hepatic stellate cell and portal area were observed under a Hitachi H-600 electron microscope. Five-micrometer-thick sections were prepared from preserved paraffin blocks stained with immunohi-stochemistry for the expression of TGF-β1 and α-SMA using the standard avidin-biotin-peroxidase complex (SABC) indirect method. The pictures were processed by colorful medical image analysis system.
In control livers, the size of hepatocytes was bigger and the nuclei situated in the center of the cells were round and large. HSCs situated in the Disse’s spaces had well-developed lipid droplets in their endoplasm. Cytoplasmic organelles were not developed.
At the end of the 3rd wk in model group, the cytoplasm of hepatocytes was loose with different size vacuoles, mitochondria were obscure and swollen with few collagen fibers proliferated in the Disse space. A few collagen fibers proliferated in the perisinusoidal Disse space and spreadout of HSCs in which nuclei became larger, rough endoplasmic reticula were rich and lipid droplet disappeared in cytoplasm. A similar hepatocyte injury was observed in IFN-α preventing group as that in model group but a mild degree of collagen proliferation and few HSCs were observed in IFN-α preventing group. Besides, apoptosis of HSC involving endoplasmic reticulum dilatation, irregular nucleus condensation of chromatin could also be seen in IFN-α preventing group.
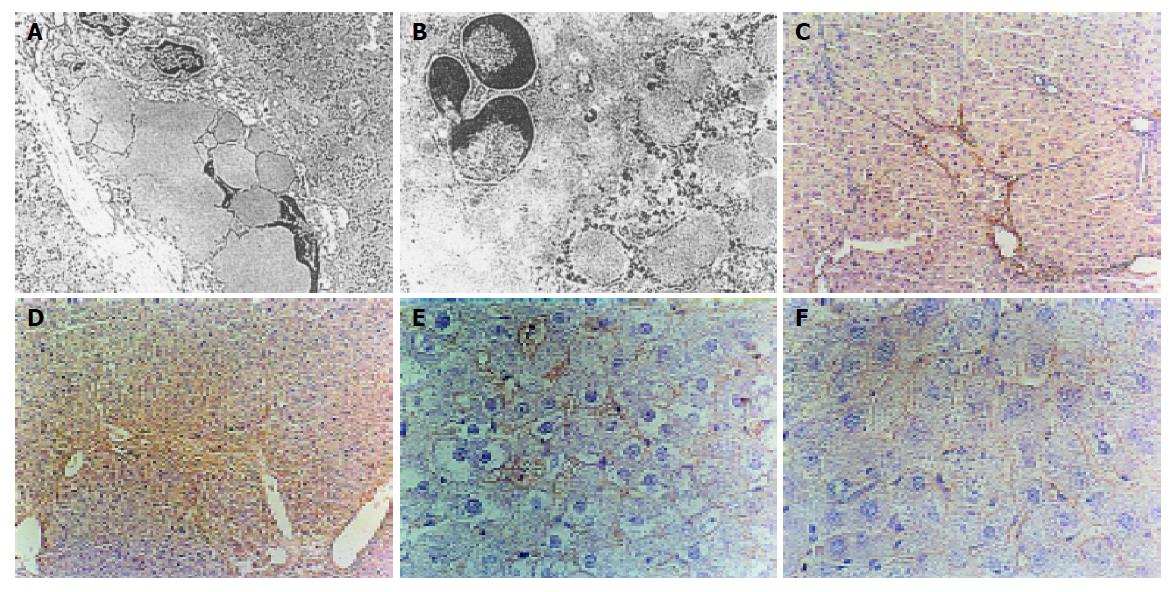
At the end of 6 wk, hepatocyte injury was worsening, cytoplasm was full of dilated rough endoplasmic reticulum. A great amount of collagens proliferated, the number of activated HSCs increased, lipid droplets decreased or even disappeared and HSC transformed into myofibroblasts (Figure 1A). In IFN-α preventing group hepatocyte structure was normal, mitochondria were still swollen. The activated HSCs were few and apoptotic HSCs significantly increased (Figure 1B).
At the end of the 12th wk, the lesion of hepatocytes alleviated and the number of activated HSCs significantly decreased in IFN-α treatment group. Early changes of apoptotic of HSCs such as side-accumulating chromatin in IFN-α treatment group, but the degree and the number of apoptotic HSCs in IFN-α treatment group were less than those in IFN-α preventing group. In 0.9% sodium chloride treatment control group, collagen fiber bundles could be seen and HSCs increased in Disse space, with little apoptosis seen.
TGF-β1 positive signals were stained brown. At the 3rd wk, TGF-β1 positive signals were situated in the sinusoidal wall, perisinusoidal cells, necrosis zone and portal area in model group. At the 6th wk, TGF-β1 expressed in inflammatory necrotic zone, connective tissue collagen bundles and fiber spatia of portal area in model group (Figure 1C). α-SMA positive cells were stained yellow-brown. In normal control group, no α-SMA positive cell was seen. At the end of the 3rd wk, α-SMA positive cells situated at portal area and fiber spatia were spindle-shaped in model group. At the end of the 6th wk, a mass of α-SMA positive cells could be seen in the dilated portal area and fiber spatia in model group (Figure 1E). With the development of hepatic fibrosis, the expression of TGF-β1 and α-SMA in liver was obvious. The correlation coefficient between them was 0.91. Compared to the model group, the expression of TGF-β1 and α-SMA in liver in IFN-α preventing group and IFN-α therapy group was significantly lower (P<0.01) (Figures 1D and 1F). Compared to sodium chloride treatment group, the expression of TGF-β1 and α-SMA in liver was significantly lower in IFN-α therapy group (P<0.01).
Hepatic fibrosis is a reversible disease, but liver cirrhosis is irreversible. Therefore, it has practical significance to seek a medicine that inhibits or delays the progression of hepatic fibrosis[4]. Cytokines can modulate the genesis of hepatic fibrosis. TGF-β1 is the key cytokine that promotes hepatic fibrosis[5]. After liver injury, TGF-β1 can promote the activation of HSCs and secrete ECM by paracrine and autocrine. There-fore, HSCs are responsible for the formation of hepatic fibrosis. The activation of HSCs is the key event of hepatic fibrosis and the activated HSCs can express α-SMA, which is the symbol of activated HSCs[6].
This study showed that IFN-α could not only prevent the formation of hepatic fibrosis but also degrade the fibrotic tissues. The probable mechanisms are as follows. IFN-α inhibits the formation of TGF-β1 and decreases the activation of HSCs. The study showed that the expression of TGF-β1, the number of HSCs and α-SMA positive cells decreased simultaneously in IFN-α preventing group and therapy group. The deposition of collagen fibers in IFN-α preventing group and therapy group was significantly less than that in model group, suggesting that IFN-α inhibits hepatic fibrosis by suppressing the expression of TGF-β1 directly and decreasing the transformation of HSCs to myofibroblasts. Therefore, IFN-α may be an inhibitor of the activation of HSCs in early stage of hepatic fibrosis. IFN-α induces apoptosis of the activated HSCs. The study showed that the decrease of activated HSCs in convalescent stage of hepatic fibrosis is mainly due to apoptosis[7-9]. The study showed the number of apoptotic of HSCs greatly increased in IFN-α preventing group compared to that in model group. Apoptosis of HSCs in the IFN-α therapy group decreased the collagen fibers in liver simultaneously, suggesting that IFN-α can prevent the waterfall effect of hepatic fibrosis by inducing apoptosis of activated HSCs. The mechanism remains to be further studied. IFN-α enhances the activity of collagenase and promotes the degradation of ECM. The deposition and degradation of hepatic fibrous tissue are a dynamic equilibrium course. The degradation depends on the activity of collagenase[10-12]. It was reported that IFN-α can enhance the activities of TIMPs, which promote the degradation of ECM[13-17], but the mechanism should be studied further.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, Calès P. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J Hepatol. 1998;29:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Chang XM, Chang Y, Jia A, Zhang YT, Li YC. Therapeutic effect of INF-α on hepatic fibrosis in rats. Zhongguo Xiandai Xiaohua Zazhi. 2003;22:12-14. [Cited in This Article: ] |
| 3. | Li D, Wang XZ. Hepatic fibrosis and INF-Aα,IL-6,IL-10. Shijie Huaren Xiaohua Zazhi. 2001;9:808-810. [Cited in This Article: ] |
| 4. | Li YC, Jia A, Chang XM, Zhang YT. Relationship between endothelin, angiotensin II, aldosterone and experimental rat liver fibrosis. Xi'an Jiaotong Daxue Xuebao. 2003;15:4648. [Cited in This Article: ] |
| 5. | Knittel T, Janneck T, Müller L, Fellmer P, Ramadori G. Transforming growth factor beta 1-regulated gene expression of Ito cells. Hepatology. 1996;24:352-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 220] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 789] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 8. | Iwamoto H, Sakai H, Tada S, Nakamuta M, Nawata H. Induction of apoptosis in rat hepatic stellate cells by disruption of integrin-mediated cell adhesion. J Lab Clin Med. 1999;134:83-89. [PubMed] [Cited in This Article: ] |
| 9. | Watanabe T, Niioka M, Ishikawa A, Hozawa S, Arai M, Maruyama K, Okada A, Okazaki I. Dynamic change of cells expressing MMP-2 mRNA and MT1-MMP mRNA in the recovery from liver fibrosis in the rat. J Hepatol. 2001;35:465-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069-11076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 11. | Hozumi A, Nishimura Y, Nishiuma T, Kotani Y, Yokoyama M. Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via NF-kappa B-mediated pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1444-L1452. [PubMed] [Cited in This Article: ] |
| 12. | Lichtinghagen R, Michels D, Haberkorn CI, Arndt B, Bahr M, Flemming P, Manns MP, Boeker KH. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J Hepatol. 2001;34:239-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, Calès P. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J Hepatol. 1998;29:263-270. [Cited in This Article: ] |
| 14. | Trim JE, Samra SK, Arthur MJ, Wright MC, McAulay M, Beri R, Mann DA. Upstream tissue inhibitor of metalloproteinases-1 (TIMP-1) element-1, a novel and essential regulatory DNA motif in the human TIMP-1 gene promoter, directly interacts with a 30-kDa nuclear protein. J Biol Chem. 2000;275:6657-6663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yeow KM, Kishnani NS, Hutton M, Hawkes SP, Murphy G, Edwards DR. Sorsby's fundus dystrophy tissue inhibitor of metalloproteinases-3 (TIMP-3) mutants have unimpaired matrix metalloproteinase inhibitory activities, but affect cell adhesion to the extracellular matrix. Matrix Biol. 2002;21:75-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Groft LL, Muzik H, Rewcastle NB, Johnston RN, Knäuper V, Lafleur MA, Forsyth PA, Edwards DR. Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br J Cancer. 2001;85:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Mengshol JA, Vincenti MP, Brinckerhoff CE. IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res. 2001;29:4361-4372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 182] [Article Influence: 7.9] [Reference Citation Analysis (0)] |