Published online Apr 21, 2005. doi: 10.3748/wjg.v11.i15.2367
Revised: August 13, 2004
Accepted: October 5, 2004
Published online: April 21, 2005
A 49-year-old woman, who had undergone hysterectomy for low-grade endometrial stromal sarcoma (ESS) 3 years ago, presented with a 2-wk history of lower abdominal pain. Barium enema and sigmoidoscopy disclosed a polypoid submucosal tumor. Histopathologic features of biopsy specimens from the lesion were similar to those of the resected uterine ESS. Under the diagnosis of metastatic ESS of the sigmoid colon, sigmoidectomy was performed. Microscopic examination demonstrated dense proliferation of spindle cells with little nuclear atypia, which were sometimes arranged in whorled pattern around abundant arterioles. Mitotic count is below 1 in 10 high-power fields. Immunohistochemically, the neoplastic cells were strongly positive for vimentin, estrogen receptor and progesterone receptor but negative for α-smooth muscle actin, S-100 protein and CD34. Thus, a final diagnosis of low-grade ESS metastasis to the sigmoid colon was made. Her postoperative course was uneventful and hormonal therapy with progestational agents is entertained.
- Citation: Asada Y, Isomoto H, Akama F, Nomura N, Wen CY, Nakao H, Murata I, Toriyama K, Kohno S. Metastatic low-grade endometrial stromal sarcoma of the sigmoid colon three years after hysterectomy. World J Gastroenterol 2005; 11(15): 2367-2369
- URL: https://www.wjgnet.com/1007-9327/full/v11/i15/2367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i15.2367
Endometrial stromal sarcoma (ESS) is a rare neoplasm comprising only 0.2% of all uterine malignancies and 15-26% of primary uterine sarcomas[1-5]. It is classified into two distinct subtypes, low-grade and high-grade, based on differences in morphological atypia and proliferative activity[6]. High-grade ESS has an aggressive nature, whereas low-grade ESS, which was formerly called endolymphatic stromal myosis, is a slowgrowing tumor with much better prognosis[6-8]. However, approximately 50% of cases of low-grade ESS develop recurrent disease and recurrences or metastases are often detected many years after initial treatment[7,9]. The common metastatic sites of low-grade ESS are the vagina, pelvis and peritoneal cavity[10]. Herein, we describe the first case of low-grade ESS metastasized to the sigmoid colon three years after initial surgery.
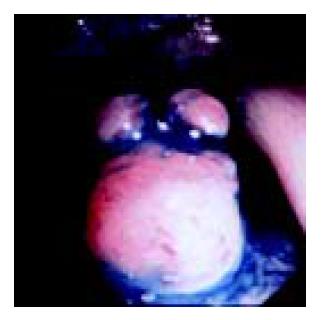
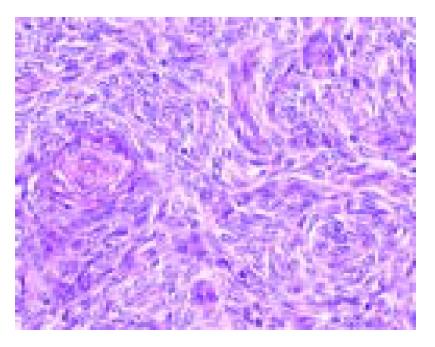
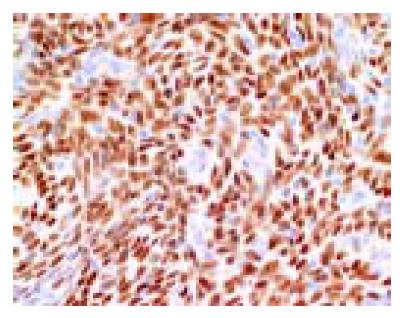
A 49-year-old woman presented with lower abdominal pain of 2-wk duration. She had undergone total hysterectomy for an alleged myoma of the uterus three years before, which was found to be low-grade ESS at postoperative pathologic examination. The family history was unremarkable. Physical examination showed lower abdominal tenderness, but no abdominal mass was palpable. Laboratory tests were normal and her serum carcinoembryonic antigen and CA 125 levels were within normal limits. Barium enema showed an irregular-shaped polypoid lesion in the sigmoid colon. Total colonoscopy disclosed an unusual polypoid tumor covered with normal-appearing mucosa with slight vessel engorgement in the sigmoid colon (Figure 1). Biopsy specimens from the lesion showed diffuse proliferation of spindle-shaped cells with little nuclear atypia, resembling the histology of previously excised uterine ESS. Computed tomograms of the chest, abdomen and pelvis, upper gastrointestinal endoscopy and barium through of the small bowel showed no abnormalities. Thus, the diagnosis was established as metastatic ESS of the sigmoid colon, and sigmoidectomy was performed. Grossly, a well-circumscribed whitish tumor 2 cm in diameter involved all layers of the colonic wall and was associated with overlying polypoid mucosa. Microscopic examination demonstrated that the tumor was composed of short fascicles or sheets of monotonous plump spindle cells with round nuclei and dispersed chromatin, which were sometimes arranged in whorled pattern around abundant arterioles (Figure 2). Mitotic count is below 1 in 10 high-power fields (HPF). Immunohistochemically, the neoplastic cells were strongly positive for vimentin, estrogen and progesterone receptors (Figure 3) but negative for -smooth muscle actin, S-100 protein and CD34. Based on these findings, a final diagnosis of low-grade ESS metastasized to the sigmoid colon was made. Her postoperative course was uneventful. She was free of symptoms at the last follow-up, 4 mo after the abdominal surgery, with no evidence of recurrence.
Clinical characteristics of low-grade ESS include a slow growth and indolent disease course with a tendency for late recurrence. One large series, the intervals before recurrence varied from 3 mo to 23 years, with a median interval of 3 years[11]. Styron et al[12], reported a patient with recurrent disease of low-grade ESS no less than 29 years after initial treatment. In our case, the metastatic tumor of the sigmoid colon was detected by colonoscopic and radiographic examination three years after hysterectomy. Patients with low-grade ESS almost develop recurrences or metastases in the vagina, pelvis and peritoneal cavity[10]. The less frequent sites were reported to be the lungs, liver, bladder, breast, heart, brain and bones[13-17]. To the best of our knowledge, however, low-grade ESS metastasized to the colon has not been previously documented.
There are two discernible subtypes of ESS, based on differences in mitotic activity: more than 10 mitotic figures for high-grade ESS and less than 10 mitotic figures per 10 HPF for low-grade ESS[6], as observed in our case. Nuclear atypia such as enlargement, increased and/or coarse chromatin and conspicuous nucleoli were absent in both the uterine and colonic lesions, distinguishing our case from high-grade ESS[6,18]. Given the gross appearance of colonic lesion at sigmoidoscopy, one can propose a variety of submucosal tumors as the differential diagnosis. Most of the mesenchymal neoplasms (leiomyoma, fibroma and schwannoma) can be immediately excluded on the basis of the histopathologic features[18]. However, the gastrointestinal stromal tumor (GIST) may be confused with low-grade ESS[18,19]. The presence of short fascicles or sheets of monotonous plump spindle cells, prominent arterioles and perivascular whorl arrangement of the tumor cells should argue against the diagnosis of GIST[18,19]. Finally, immunohistochemical analysis is useful in distinguishing between these entities, as GIST is well known to stain diffusely for CD34 and CD117[20] and ESS exclusively for estrogen receptor and progesterone receptor, reflecting the high sensitivity of low-grade ESS to sex-steroid hormones[2,3,5,18,21,22]. Again, low-grade ESS arising in the gastrointestinal endometriosis has been rarely documented[18,19,23]. According a review in literature by Cho et al[19], most of the lesions were located in the rectosigmoid colon, which is an area of bowel having the highest incidence of endometriosis[23]. Our patient has not suffered from endometriosis and there was no evidence of underlying endometriosis in the surgical specimens. Thus, the definite diagnosis of low-grade ESS metastasized to the sigmoid colon was made in this case.
Surgical excision is the major therapeutic procedure for the primary low-grade ESS, but the standard treatment for its recurrent disease including radiotherapy and chemotherapy has not been established[16,24,25]. Some surgeons recommended surgical cytoreduction of the ESS tumors when the extrau-terine lesions were present[25,26]. On the other hand, Mansi et al[9], asserted that progesterone therapy should be the treatment of first choice for relapsed low-grade ESS, because there was resolution or stabilization of recurrent or metastatic disease in more than 50% of patients treated with progestational agents[12,16,27]. In this regard, immunoreactivity for estrogen receptor and progesterone receptor should be routinely assessed in low-grade ESS, in particular manifested by recurrence[28]. Adjunct progesterone therapy is now entertained in our patient, since the primary and metastatic tumors showed strong and diffuse staining against anti-estrogen receptor and progesterone receptor antibodies. Considering highly recurrent nature of low-grade ESS, sometimes many years later[7,9,11,12,15,22], a life-long follow-up is necessary for this woman.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Koss LG, Spiro RH, Brunschwig A. Endolymphatic stromal sarcoma. Surg Gynecol Obstet. 1965;121:531-537. [PubMed] [Cited in This Article: ] |
| 2. | Farhood AI, Abrams J. Immunohistochemistry of endometrial stromal sarcoma. Hum Pathol. 1991;22:224-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 107] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Gil-Benso R, López-Ginés C, Navarro S, Carda C, Llombart-Bosch A. Endometrial stromal sarcomas: immunohistochemical, electron microscopical and cytogenetic findings in two cases. Virchows Arch. 1999;434:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Kempson RL, Bari W. Uterine sarcomas. Classification, diagnosis, and prognosis. Hum Pathol. 1970;1:331-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 182] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Sabini G, Chumas JC, Mann WJ. Steroid hormone receptors in endometrial stromal sarcomas. A biochemical and immunohistochemical study. Am J Clin Pathol. 1992;97:381-386. [PubMed] [Cited in This Article: ] |
| 6. | Norris HJ, Taylor HB. Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer. 1966;19:755-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 7. | Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990;14:415-438. [PubMed] [Cited in This Article: ] |
| 8. | Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, Urgesi A, Lissoni A, Losa G, Fanucchi A. Endometrial stromal sarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;63:247-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 151] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Mansi JL, Ramachandra S, Wiltshaw E, Fisher C. Endometrial stromal sarcomas. Gynecol Oncol. 1990;36:113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Montag TW, Manart FD. Endolymphatic stromal myosis: surgical and hormonal therapy for extensive venous recurrence. Gynecol Oncol. 1989;33:255-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Piver MS, Rutledge FN, Copeland L, Webster K, Blumenson L, Suh O. Uterine endolymphatic stromal myosis: a collaborative study. Obstet Gynecol. 1984;64:173-178. [PubMed] [Cited in This Article: ] |
| 12. | Styron SL, Burke TW, Linville WK. Low-grade endometrial stromal sarcoma recurring over three decades. Gynecol Oncol. 1989;35:275-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Yoonessi M, Hart WR. Endometrial stromal sarcomas. Cancer. 1977;40:898-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Hart WR, Yoonessi M. Endometrial stromatosis of the uterus. Obstet Gynecol. 1977;49:393-403. [PubMed] [Cited in This Article: ] |
| 15. | Günhan-Bilgen I, Memis A, Ustün EE, Ozdemir N. Breast metastasis from low-grade endometrial stromal sarcoma after a 17-year period. Eur Radiol. 2002;12:3023-3025. [PubMed] [Cited in This Article: ] |
| 16. | Matsuura Y, Yasunaga K, Kuroki H, Inagaki H, Kashimura M. Low-grade endometrial stromal sarcoma recurring with multiple bone and lung metastases: report of a case. Gynecol Oncol. 2004;92:995-998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Yokoyama Y, Ono Y, Sakamoto T, Fukuda I, Mizunuma H. Asymptomatic intracardiac metastasis from a low-grade endometrial stromal sarcoma with successful surgical resection. Gynecol Oncol. 2004;92:999-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Mourra N, Tiret E, Parc Y, de Saint-Maur P, Parc R, Flejou JF. Endometrial stromal sarcoma of the rectosigmoid colon arising in extragonadal endometriosis and revealed by portal vein thrombosis. Arch Pathol Lab Med. 2001;125:1088-1090. [PubMed] [Cited in This Article: ] |
| 19. | Cho HY, Kim MK, Cho SJ, Bae JW, Kim I. Endometrial stromal sarcoma of the sigmoid colon arising in endometriosis: a case report with a review of literatures. J Korean Med Sci. 2002;17:412-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11:728-734. [PubMed] [Cited in This Article: ] |
| 21. | Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 2003;90:170-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Satoh Y, Ishikawa Y, Miyoshi T, Mukai H, Okumura S, Nakagawa K. Pulmonary metastases from a low-grade endometrial stromal sarcoma confirmed by chromosome aberration and fluorescence in-situ hybridization approaches: a case of recurrence 13 years after hysterectomy. Virchows Arch. 2003;442:173-178. [PubMed] [Cited in This Article: ] |
| 23. | Mostoufizadeh M, Scully RE. Malignant tumors arising in endometriosis. Clin Obstet Gynecol. 1980;23:951-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 180] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Pautier P, Genestie C, Fizazi K, Morice P, Mottet C, Haie-Meder C, Le Cesne A, Lhommé C. Cisplatin-based chemotherapy regimen (DECAV) for uterine sarcomas. Int J Gynecol Cancer. 2002;12:749-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Weitmann HD, Kucera H, Knocke TH, Pötter R. Surgery and adjuvant radiation therapy of endometrial stromal sarcoma. Wien Klin Wochenschr. 2002;114:44-49. [PubMed] [Cited in This Article: ] |
| 26. | Geas FL, Tewari DS, Rutgers JK, Tewari KS, Berman ML. Surgical cytoreduction and hormone therapy of an advanced endometrial stromal sarcoma of the ovary. Obstet Gynecol. 2004;103:1051-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Tsukamoto N, Kamura T, Matsukuma K, Imachi M, Uchino H, Saito T, Ono M. Endolymphatic stromal myosis: a case with positive estrogen and progesterone receptors and good response to progestins. Gynecol Oncol. 1985;20:120-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Reich O, Regauer S, Urdl W, Lahousen M, Winter R. Expression of oestrogen and progesterone receptors in low-grade endometrial stromal sarcomas. Br J Cancer. 2000;82:1030-1034. [PubMed] [Cited in This Article: ] |