Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2162
Revised: November 21, 2004
Accepted: December 9, 2004
Published online: April 14, 2005
AIM: To study the clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer.
METHODS: To investigate the expression of p53 and mdm2 in pancreatic cancer by immunohistochemistry, and the relationships between the p53 and mdm2 protein expression and clinicopathological parameters in pancreatic cancer.
RESULTS: The positive expression of p53 protein was found in 40 of 59 patients (67.8%) and that of mdm2 protein in 17 of 59 patients (28.8%). No obvious relationships were found between p53 as well as mdm2 expression and sex, tumor site, TNM staging and histological differentiation. p53 expression was increased in patients younger than 65 years old, while mdm2 had no relationship with age. The survival time of the patients with the positive expression of p53 and mdm2 proteins was obviously shorter than the other groups.
CONCLUSION: Both p53 and mdm2 presented relatively high expression in human pancreatic cancer. The overexpression of p53 and mdm2 might reflect the malignant proliferation of pancreatic cancer and their co-expression might be helpful to evaluate the prognosis of the patients with pancreatic cancer.
- Citation: Dong M, Ma G, Tu W, Guo KJ, Tian YL, Dong YT. Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J Gastroenterol 2005; 11(14): 2162-2165
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2162
Pancreatic cancer is a gastrointestinal neoplasm with high malignancy and poor prognosis. Incidence of pancreatic cancer has shown the increasing trend in recent years, but the therapeutic efficacy is still not satisfying. That is related to the pancreas’ deep location, lack of specific early symptoms, difficult early diagnosis and scarce chance of surgical resection; even for the surgical cases, the 1-, 3- and 5-year cumulative survival rates were 49.8%, 16.8% and 9.6%, respectively[1].
With the development of techniques, molecular biology research has indicated that p53 tumor suppressor gene plays an important role in DNA transcription, cell growth and proliferation, DNA repair and various metabolic processes. p53 abnormalities such as gene mutation and depletion can lead to the altered intracellular signal transduction pathways as well as loss of the regulation of cell growth, apoptosis, and DNA repair, which are responsible for carcinogenesis. Previous report showed that p53 gene mutation rate in pancreatic cancer is as high as 50-70%[2,3]. p53 protein expression and gene mutation may indicate the prognosis of pancreatic cancer, and their expression level might be useful in the determination of surgical therapy outcome and clinical prognosis[4]. But, controversy still remains in this point at present. mdm2, murine double minute gene 2, is an oncogene (the corresponding human homologous gene is hdm2). mdm2 protein (homologous protein in human is called hdm2 protein) can be combined with p53 to inhibit p53 function of growth supervision, leading to cell overgrowth into tumor. Therefore, we detected the expression of p53 and mdm2 in primary invasive ductal carcinoma (IDC) of the pancreas by immunohistochemistry, analyzed their relationships to clinicopathological parameters and then investigated the influence on the biological activity of pancreatic cancer.
Fifty-nine well-documented surgically resectable specimens of IDCs of the pancreas were obtained in The First Affiliated Hospital of China Medical University from May 1978 to May 1997. During this period, the main method for treating pancreatic cancer was standard pancreatioduodenectomy, although the diagnostic procedure has relatively improved. The specimens were processed routinely by 40 g/L formaldehyde fixation and paraffin-embedment. All the cases were confirmed as IDCs of the pancreas pathologically. There were 21 male cases and 38 female cases. Ages of the patients ranged from 23 to 76 years old (47 cases <65 years old; 12 cases ≥65 years old). Based on TNM staging standard established by International Union Against Cancer (UICC), 4 patients were in stage I, 8 were in stage II, 42 were in stage III, and 5 were in stage IV. Differentiation degree: 19 cases were well differentiated, 21 cases moderately differentiated and 19 cases poorly differentiated. Average survival time after surgery was 11.2 mo and still two cases survived at the end of follow-up. Because a few cases were performed with radical pancreatioduodenectomy, we did not analyze specially the effect of this style of operation on prognosis of the patients. In addition, five patients in stage IV underwent standard pancreatioduodenectomy from achievement data, but not vascular excision. Because we cannot get whether these tumor margins were checked in operation note or not, it is different to evaluate the effect free of tumor margins on prognosis.
Immunostaining was performed using the streptavidin-biotin technique (SAB method). p53 monoclonal antibody (Ab-6) and mdm2 (Ab-1) were purchased from Dako Company. According to the manufacturer’s instruction, antibody dilutions at various working concentration were prepared: p53 (Ab-6), 1:20; mdm2 (Ab-1), 1:100.
The 4-μm sections were deparaffinized with xylene thrice for 3-5 min each, dehydrated in a gradient series of alcohol thrice (100%, 95% and 45% alcohol), and rinsed by PBS. Each section was covered with 0.3% peroxyacetic acid for 15 min to block endogenous peroxidase activity, microwaved for antigen retrieval (800 W, 5 min×3 min), and cooled in the room temperature for 40 min. Non-specific binding sites were blocked by 10% normal rabbit serum for 10 min. These sections were first incubated with primary antibody for 2 h at room temperature, and then rinsed twice with PBS. This is followed by incubation with a secondary antibody for 15 min at 37 °C as well as another rinsed twice with PBS. Slides were then treated with streptavidin-peroxidase reagent for 10 min and rinsed with PBS twice. The sections were visualized with 3,3’-diaminobenzidine (DAB) for 5 min, counterstained with hematoxylin and mounted for observation under microscope.
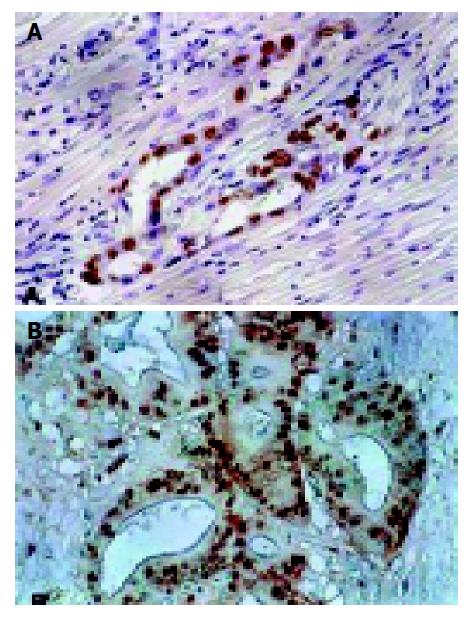
Nuclear staining of p53 and mdm2 protein was shown as brown granules (Figure 1). Positive result was defined as 10% or more than 10% of the tumor cells showing positive staining.
SAS 8.0 was used. p53 and mdm2 interaction as well as their correlations with clinicopathological parameters was performed by χ2 test. Group comparison was analyzed by analysis of variance. Statistical analyses for cumulative survival rate, survival time difference and multiplicity were performed using Kaplan-Meier method, log-rank test and Cox proportional hazards model, respectively. Significant differences were accepted at P<0.05.
p53 protein expression rate was 67.8% (40/59); but mdm2 protein expression rate was 28.8% (17/59). p53 and mdm2 protein expression rate did not correlate with sex, tumor site, TNM staging and differentiation rate. p53 expression was relatively high among the group with age <65 years old (χ2 = 4.711, P<0.05). There was no relationship between mdm2 expression and age (Table 1).
| Parameters | No. of patients(n = 59) | Number expressing (%) | ||
| p53(+) (n = 40) | mdm2(+) (n = 17) | |||
| Gender | Male | 38 | 25 (65.8) | 10 (26.3) |
| Female | 21 | 15 (71.4) | 7 (33.3) | |
| Age (yr) | <65 | 47 | 35 (74.5) | 13 (27.7) |
| ≥65 | 12 | 5 (41.7) | 4 (33.3) | |
| Site | Head | 52 | 34 (65.4) | 16 (30.8) |
| Body/tail | 7 | 6 (85.7) | 1 (14.3) | |
| TNM stage | I | 4 | 2 (50) | 0 (0) |
| II | 8 | 4 (50) | 0 (0) | |
| III | 42 | 34 (81) | 15 (35.7) | |
| IV | 5 | 4 (80) | 2 (40) | |
| Grade | Well | 19 | 14 (73.7) | 4 (21.1) |
| Moderate | 21 | 12 (57.1) | 9 (42.9) | |
| Poor | 19 | 14 (73.7) | 4 (21.1) | |
p53 expression rate was 88.2% (15/17) in the mdm2 expression positive cases, and 59.5% (25/42) in the mdm2 expression negative cases, indicating that there was correlation between the p53 and mdm2 protein expression (χ2 = 4.57, P = 0.0325).
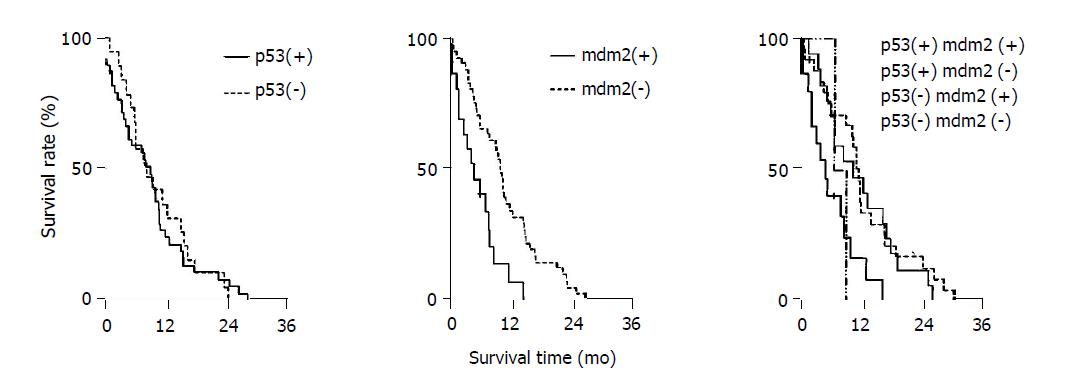
As shown in Table 2, median survival time of p53(+) and mdm2(+) group was 7.4 mo, p53(+) and mdm2(-) group 13.5 mo, p53(-) and mdm2(+) group 9.2 mo, p53(-) and mdm2(-) group 12.8 mo. Kaplan–Meier method was used for analyzing cumulative survival rate (Figure 2). Group comparison was analyzed by log-rank test, indicating that the median survival time of various groups had significant difference (χ2 = 11, P<0.05). p53(+) and mdm2(+) group had shorter survival time as compared with other groups.
Cox proportional hazards model was applied in multifactor analysis (p53, mdm2, clinicopathological parameters and survival time after surgery), indicating various factors such as sex, age, tumor site, TNM staging, differentiation rate, and p53 had no correlation with survival time after surgery, but mdm2 was an exception (P<0.05, Table 3).
| Variable | Parameter estimate (SE) | Conditional risk ratio (95% confidence limits) | P (χ2) |
| mdm2 | 0.969 (0.324) | 2.636 (1.397-4.975) | 0.003 |
| Age (yr) | 0.210 (0.013) | 1.021 (0.996-1.047) | 0.101 |
| TNM | 0.279 (0.223) | 1.322 (0.853-2.048) | 0.212 |
| Grade | 0.207 (0.193) | 1.229 (0.842-1.795) | 0.285 |
| Site | -0.468 (0.469) | 0.627 (0.250-1.570) | 0.318 |
| p53 | 0.336 (0.345) | 1.399 (0.712-2.751) | 0.330 |
| Gender | -0.010 (0.352) | 0.990 (0.496-1.974) | 0.977 |
p53 tumor suppressor gene located at 17q13.1, which can induce cell apoptosis. Wild-type p53 protein inhibits cell proliferation, halts cell division at the G1 checkpoint, and facilitates the injured DNA repair. p53 protein can induce cell apoptosis to prevent the mutated DNA passage to the next generation in case of the failed DNA repair. Due to the loss of cell supervision of p53 protein after mutation, cell is susceptible to entry of S phase with injured DNA and the genetic instability is the source of gene mutation and chromosomal aberration, leading to cell malignant change and tumor formation. In our experiment, p53 protein expression rate was 67.8%. Almost all the detected p53 protein is mutated because the extremely short half-life of wild-type p53 protein makes the immunohistochemical detection invalid. This expression rate is consistent with the 50-70% of p53 mutation rate in pancreatic cancer according to previous reports[2].
mdm2, a newly discovered oncogene, is located at 12q13.14. The major function of mdm2 is to inhibit the transcription activation by p53 as well as to prevent carcinogenesis. As the target gene of p53 transcription, mdm2 can combine with p53 to form a refined feedback regulation loop. Wild-type p53 gene induces the high expression of mdm2 protein, which, in turn, inhibits p53 transcription activity and strictly controls p53 protein level. mdm2 overexpression can block the p53-mediated transactivation, depriving p53 gene of antineoplastic activity[5]. mdm2 gene amplification has been found in 36% of all types of sarcomas, 10% of well-differentiated glioma as well as esophageal cancer, neuroblastoma, anaplastic astrocytoma[6]. Our study has proved that p53 protein expression rate was 88.2% (15/17) in mdm2 positive cases and 59.5% (25/42) in mdm2 negative cases, indicating the correlation between the two proteins.
Cox proportional hazards model was applied in multifactor analysis (p53, mdm2, clinicopathological parameters and survival time after surgery), indicating only mdm2 had correlation with survival time after surgery. Patients with negative mdm2 expression had longer survival time after surgery. However, various factors including sex, age, tumor site, TNM staging, differentiation rate and p53 had no correlation with survival time after surgery. Whether p53 expression in pancreatic cancer is related to prognosis is still under debate, although our data showed that the survival time after surgery for the group with positive p53 expression was shorter than that for the group with negative p53 expression (Table 2). Some authors believe that p53 expression correlates with poor prognosis and it has been reported that p53 expression and p53 gene mutation can serve as an indicator of prognosis[7,8]. The reason for this confusing condition is still unknown. It may be related to the antibody choice, specimen process and preservation method[9,10]. Another possible reason is that p53 protein cannot completely reflect the p53 gene expression changes. For example, immunohistochemical staining might not detect p53 protein expression although p53 gene abnormalities (deletion mutation, frame shift mutation, nonsense mutation) or mdm2 overexpression are present. In these cases, there is no p53 protein expression but p53 gene expression. Thus, we believe that p53 protein expression cannot truly reflect p53 gene change, which is related to poor prognosis.
Although multifactor analysis by Cox proportional hazards model indicated that only mdm2 correlates to the survival time after surgery, different combination of p53 protein and mdm2 protein may be related to the prognosis. As shown in Table 2, p53(+) and mdm2(+) group had shorter survival time as compared with other groups, indicating that overexpression of p53 and mdm2 protein may reflect malignant proliferation of pancreatic cancer and combined detection of the two proteins may be beneficial for the prediction of prognosis. The mdm2 oncogene product forms a complex with the p53 protein and affects normal p53 function[9]. However, the cases in this study were too small to draw a definitive conclusion. Further studies in increased number of patients using a rigorous research design are necessary in the future.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer. 1995;76:1671-1677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 2. | Dong M, Nio Y, Sato Y, Tamura K, Song MM, Tian YL, Dong YT. Comparative study of p53 expression in primary invasive ductal carcinoma of the pancreas between Chinese and Japanese. Pancreas. 1998;17:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Yuan RW, Ding Q, Jiang HY, Qin XF, Zou SQ, Xia HS. Bcl-2, P53 protein expression and apoptosis in pancreatic cancer. Shijie Huren Xiaohua Zazhi. 1999;7:851-854. [Cited in This Article: ] |
| 4. | Inoue S, Tezel E, Nakao A. Molecular diagnosis of pancreatic cancer. Hepatogastroenterology. 2001;48:933-938. [PubMed] [Cited in This Article: ] |
| 5. | Lev Bar-Or R, Maya R, Segel LA, Alon U, Levine AJ, Oren M. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc Natl Acad Sci USA. 2000;97:11250-11255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 359] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Bueso-Ramos CE, Manshouri T, Haidar MA, Huh YO, Keating MJ, Albitar M. Multiple patterns of MDM-2 deregulation in human leukemias: implications in leukemogenesis and prognosis. Leuk Lymphoma. 1995;17:13-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Zhang SY, Ruggeri B, Agarwal P, Sorling AF, Obara T, Ura H, Namiki M, Klein-Szanto AJ. Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med. 1994;118:150-154. [PubMed] [Cited in This Article: ] |
| 8. | Sinicrope FA, Evans DB, Leach SD, Cleary KR, Fenoglio CJ, Lee JJ, Abbruzzese JL. bcl-2 and p53 expression in resectable pancreatic adenocarcinomas: association with clinical outcome. Clin Cancer Res. 1996;2:2015-2022. [PubMed] [Cited in This Article: ] |
| 9. | Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237-1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2174] [Cited by in F6Publishing: 2260] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 10. | Frebourg T, Friend SH. The importance of p53 gene alterations in human cancer: is there more than circumstantial evidence? J Natl Cancer Inst. 1993;85:1554-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |