Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.2032
Revised: July 18, 2004
Accepted: November 4, 2004
Published online: April 7, 2005
We report an autopsy case of acute pancreatitis with a high serum IgG4 concentration complicated by systemic amyloid A amyloidosis and rheumatoid arthritis (RA). The patient was a 42-year-old Japanese female with a 22-year history of rheumatoid arthritis. She was diagnosed with myasthenia gravis when she was 31-year old. At the onset of pancreatitis, the patient was anti-nuclear antibody-positive, and had high serum gamma globulin and IgG4 levels. Dexamethasone and conventional therapy induced clinical remission and significantly decreased the serum IgG4 and gamma globulin. However, despite the decreased disease parameters, the patient developed a bleeding pseudocyst and died of cardiac failure. In the autopsy examination, it was determined that pancreatitis was probably caused by ischemia due to vascular obstruction caused by amyloid deposition in the pancreas. Even though acute pancreatitis is a rare complication in RA patients, we speculate that an autoimmune pancreatitis-related mechanism and ischemia due to vascular obstruction by amyloid deposition might be attributable to a single source that leads to acute pancreatitis in our particular case.
- Citation: Ichikawa T, Nakao K, Hamasaki K, Ohkubo K, Toriyama K, Eguchi K. An autopsy case of acute pancreatitis with a high serum IgG4 complicated by amyloidosis and rheumatoid arthritis. World J Gastroenterol 2005; 11(13): 2032-2034
- URL: https://www.wjgnet.com/1007-9327/full/v11/i13/2032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.2032
Acute pancreatitis is a relatively mild disease and has a low-associated morbidity. However, 4-7% of acute pancreatitis patients suffer from a severe illness that has been associated with mortality rates approaching 20-50%[1]. Etiologies of acute pancreatitis are bile duct stones, alcohol abuse, various toxins, drugs, other obstructive causes, metabolic abnormality, trauma, ischemia, infection, autoimmune disease and idiopathic causes[2]. In previous reports, pancreatitis occurring simultaneously with rheumatoid arthritis (RA) has been associated with Sjögren’s syndrome (SjS)[3], anti-RA drugs[4], and amyloidosis[5]. It has been reported that a patient who developed amyloidosis following ankylosing spondylitis died of pancreatitis[6]. Occasionally, autoimmune disease is complicated by pancreatitis, which has led to the concept of an autoimmune-related pancreatitis and autoimmune pancreatitis has been correlated with other autoimmune diseases such as SjS, primary sclerosing cholangitis, ulcerative colitis, systemic lupus erythematosus[7] and myasthenia gravis[8]. Patients with autoimmune pancreatitis/sclerosing pancreatitis have several characteristic autoantibodies[7], and high serum gamma globulin and IgG4 concentrations[9]. In this paper, we report a rare case of acute pancreatitis in a patient with RA, who had a high serum IgG4 concentration and a condition complicated by systemic AA amyloidosis.
The patient was a 42-year-old Japanese female with a 22-year history of RA. In 1989, she was diagnosed with myasthenia gravis. In 1992, a stomach mucosal biopsy was carried out and amyloid deposition was confirmed. In December 1998, she had occasional epigastralgia after food intake. She had no history of habitual alcohol consumption and she had taken low-dose oral prednisolone (7.5 mg/d).
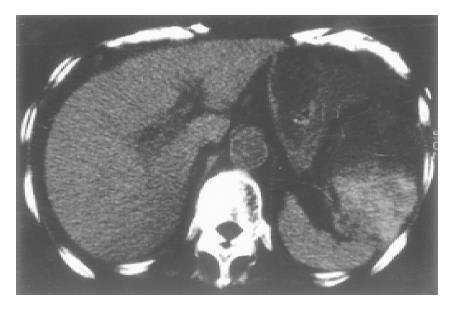
The patient presented with clinical pancreatitis with severe epigastralgia, back pain, nausea and vomiting and she was admitted to our hospital on January 7, 1999. On admission, she had bilateral joint deformity of the elbow, wrist, MP, PIP and DIP joints but no arthralgia and myasthenia. She had a fever of 38 °C, tenderness of the epigastrium region and diminished bowel sound. Laboratory data showed the following values: 8058 IU/L serum amylase, 1800 ng/dL serum elastase 1, 1.946 g/dL gamma globulin, 1/160 ANA and 156 mg/dL IgG4. Ultrasound (US) examination and abdominal computed tomography (CT) scan revealed a slightly thickened gallbladder wall with a small stone and without biliary duct dilatation, and mild swelling of the pancreatic head consistent with effusion of the peripancreatic space. A left pleural effusion was identified by chest x-ray. Treatment for pancreatitis with an intravenous protease inhibitor, antibiotics and intramuscular dexamethasone (2 mg/d) was commenced on admission. On the 9th hospital day, the patient’s condition became complicated with congestive heart failure and bacterial pneumonia. From a cardiac US examination, we suspected a case of cardiac amyloidosis. On the 25th d, abdominal pseudocysts were found in hilum of the spleen and mesentery of the transverse colon by abdominal CT (Figure 1). The severity of abdominal symptoms was decreased by the treatment for pancreatitis and additionally, serum IgG4 and gamma globulin concentrations were decreased (31 mg/dL and 0.665 g/dL, respectively) on the 67th d. However, serum amylasemia continued to be severe, and we tried a new therapy using an intrasubcutaneous somatostatin analogue on the 75th d. Following this treatment, the serum amylase level gradually decreased and the patient started to eat on the 119th d.
On the 103rd d, hemorrhage occurred in a pseudocyst of the anterior spleen region. The patient complained of epigastralgia, but the hemorrhage was diminished by conservative treatment. However, re-bleeding in the same pseudocyst occurred on the 150th d, and anemia and hemorrhagic shock developed. The patient’s condition recovered slightly following blood transfusion, but she then developed congestive heart failure. Several treatments for heart failure did not seem to be effective and the patient developed massive fresh blood stools and died on the 170th hospital day. We were permitted to perform an autopsy.
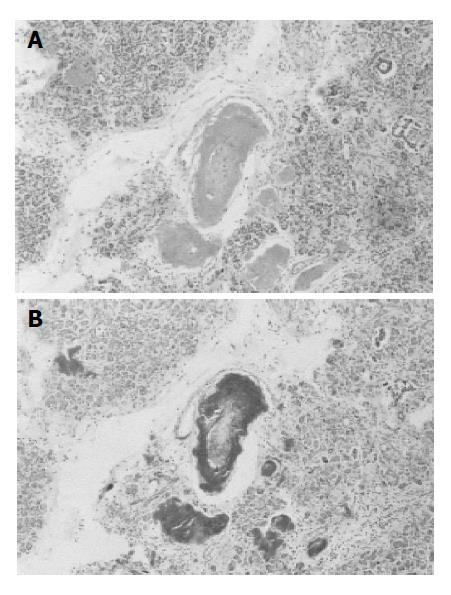
The autopsy findings were as follows: A marked amyloid deposition (AA type) was found mainly in the vascular wall with stenotic and obstructive changes, and in stroma of the heart, kidney, gastro-intestinal tract, liver, skin, pancreas and other organs. Pancreatic pseudocysts of 5 and 10 cm in diameter retaining blood and necrotic tissue were present in the mesentery and peritonitis with yellowish turbid ascites was identified in the mesentery and retroperitonea. In the pancreas, extensive fibrosis with lymphocyte infiltration, atrophy of acinar cells, dense pancreatic juice retention and dilatation of the pancreatic duct were observed. The small arteries and arterioles in the pancreas were obstructed and narrowed by marked amyloid deposition on the artery walls (Figure 2). There was no inflammatory cell infiltration in the common bile duct of the pancreas. Heart enlargement (500 g) occurred and the right and left ventricle extension was most unsatisfactory. Histopathological examination revealed diffuse amyloid deposition in myocardium and vascular wall, and an intramural thrombus in the right atrium. Dense amyloid deposition in the stroma and vascular wall was observed throughout the gastrointestinal tract. Multiple ulceration of the rectum reached the muscle layer. These results confirmed cardiac failure with systemic amyloidosis as the cause of death of the patient.
We have reported an autopsy case of necrotizing pancreatitis in a RA patient. In a postmortem histopathological examination, amyloid deposition was observed on walls of the small arteries and arterioles in the pancreas. Because we did not observe inflammation in the pancreatic common bile duct, the etiology of acute pancreatitis was not bile duct stones. Several cases of pancreatitis in RA patients have previously been reported[5,10], and in one of these cases, amyloidosis was found. Oishi et al[5], described that fatal pancreatitis is associated with systemic amyloidosis in RA patients. In their report, a pancreatic biopsy is performed and AA amyloid deposition is observed on the vascular wall of pancreatic arteries and arterioles. It was speculated that the impairment of microcirculation of the pancreatic vasculature reduces the blood perfusion and causes more rapid deterioration, compared to the natural course of acute pancreatitis[5]. However, acute pancreatitis is a rare complication in RA patients, and we strongly suggest that pancreatitis is likely to be caused by ischemia due to vascular obstruction by amyloid deposition in the pancreas, which is brought about by RA-induced secondary AA amyloidosis.
Occasionally, autoimmune disease has been reported to be complicated by pancreatitis, which has led to the concept of an autoimmune-related pancreatitis[7]. In our case, some of the clinical characteristics (presence of an autoantibody, increased levels of gamma globulin and IgG4, effectiveness of steroid therapy and association with another autoimmune disease, myasthenia gravis) correspond to autoimmune pancreatitis. Hamano et al[9], reported that a high serum IgG4 concentration provides a useful means of distinguishing autoimmune pancreatitis/sclerosing pancreatitis from other diseases of the pancreas and biliary tract. In our case, dexamethasone and conventional therapy induced clinical remission and significantly decreased the serum IgG4 and gamma globulin levels. These results indicate that acute pancreatitis might be triggered by the same mechanism of autoimmune pancreatitis that was present in our case. However, acute pancreatitis and pancreatic cysts are unusual findings in autoimmune pancreatitis[7] and autopsy examination does not definitely indicate autoimmune pancreatitis. Hence, we speculate that an autoimmune pancreatitis-related mechanism may exist, which might be attributable to a single source that can also lead to acute pancreatitis.
In summary, acute pancreatitis is a rare complication in RA patients, but RA patients with systemic AA amyloidosis can develop acute pancreatitis from ischemia due to vascular obstruction by amyloid deposition. Such cases might be considered to be autoimmune-related pancreatitis because of the presence of autoantibodies, a high serum IgG4 concentration and an association with other autoimmune diseases.
| 1. | Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol. 2000;30:343-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Bank S, Singh P, Pooran N, Stark B. Evaluation of factors that have reduced mortality from acute pancreatitis over the past 20 years. J Clin Gastroenterol. 2002;35:50-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Grindulis KA, Roy S. Renal and pancreatic calcification in rheumatoid arthritis with Sjögren's syndrome. Br J Rheumatol. 1987;26:212-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Eisemann AD, Becker NJ, Miner PB, Fleming J. Pancreatitis and gold treatment of rheumatoid arthritis. Ann Intern Med. 1989;111:860-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Oishi K, Wada J, Nagake Y, Hida K, Hashimoto H, Hayakawa N, Kashihara N, Makino H. Fatal pancreatitis associated with systemic amyloidosis in a rheumatoid arthritis patient. J Med. 2000;31:303-310. [PubMed] [Cited in This Article: ] |
| 6. | Fujito T, Inoue T, Hoshi K, Hatano H, Kamishirado H, Takayanagi K, Hayashi T, Morooka S, Takabatake Y, Uehara Y. Systemic amyloidosis following ankylosing spondylitis associated with congestive heart failure. A case report. Jpn Heart J. 1995;36:681-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 305] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Colaut F, Toniolo L, Sperti C, Pozzobon M, Scapinello A, Sartori CA. Autoimmune-like pancreatitis in thymoma with myasthenia gravis. Chir Ital. 2002;54:91-94. [PubMed] [Cited in This Article: ] |
| 9. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2026] [Cited by in F6Publishing: 1802] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 10. | Bély M, Apáthy A, Pintér T, Ratkó I. Pancreatitis in rheumatoid arthritis found in autopsy material. Morphol Igazsagugyi Orv Sz. 1989;29:213-221. [PubMed] [Cited in This Article: ] |