Published online Mar 21, 2005. doi: 10.3748/wjg.v11.i11.1705
Revised: September 9, 2004
Accepted: November 19, 2004
Published online: March 21, 2005
AIM: To study the expression of hypoxia-inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF) in hepatocellular carcinoma (HCC) and the impact on neovascularization and survival.
METHODS: Expressions of HIF-1α, VEGF and microvessel density (MVD) are studied through immunohistochemistry in 36 cases of HCC and the corresponding paraneoplastic tissue and 6 cases of normal liver tissue. The relationship of the expressions of HIF-1α and VEGF with the clinicopathological data and survival are analyzed.
RESULTS: The positive rate of VEGF in HCC was 32/36, which is significantly higher than that in paraneoplastic tissue and normal liver tissue (P<0.05). The expression of HIF-1α in HCC tissue is 24/36, also higher than that in paraneoplastic tissue and normal liver tissue (P<0.05). The expression of VEGF and HIF-1α in HCC with microscopic venous invasion is significantly higher than that in HCC without microscopic venous invasion (P<0.05). Spearman correlation analysis does not only show the expression of HIF-1α as correlated with the expression of VEGF (rs = 0.459, P<0.01), but it also shows the expression of HIF-1α and VEGF as correlated with MVD (rs = 0.412 and 0.336, respectively, P<0.05). The differences of the survival rates among VEGF positive group and VEGF negative group are significant (P<0.05), whereas the differences of the survival rates among the HIF-1α negative group and positive group are not significant (P>0.05).
CONCLUSION: HIF-1α plays important roles in neovascularization in HCC possibly through regulation of VEGF transcription.
- Citation: Huang GW, Yang LY, Lu WQ. Expression of hypoxia-inducible factor 1α and vascular endothelial growth factor in hepatocellular carcinoma: Impact on neovascularization and survival. World J Gastroenterol 2005; 11(11): 1705-1708
- URL: https://www.wjgnet.com/1007-9327/full/v11/i11/1705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i11.1705
Hepatocellular carcinoma (HCC) is one of the most common malignancies in China. Owing to the improvement of surgical technique and early diagnostic methods, the resection rate of HCC has increased[1,2]. However, the postoperative relapse and metastatic rate remains high[3]. Neovascularization may play important roles in the relapse and metastasis of HCC[4-6].
Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric transcriptional factor composed of the two basic-helix-loop-helix (bHLH) -PAS α and β subunits[7,8]. HIF-1α is the unique, O2-regulated subunit that determines HIF-1 activity. A series of genes and proteins that may increase the survival of tumor cells under hypoxia conditions, including vascular endothelial growth factor (VEGF), insulin-like growth factor, inducible nitric oxide synthase, platelet-derived endothelial growth factor, glucose transporter 1, lactate dehydrogenase, erythropoietin and nitric oxide synthase gene, are regulated by HIF-1α[9-12]. Thus, HIF-1α may play important roles in tumor progression.
VEGF is a potent proangiogenic agents. There is a specific binding site with HIF-1α in the initiating area of VEGF genes[13-15]. Thus, HIF-1α may also be important in the regulation of neovascularization of malignant tumors.
The objective of this clinical study is to investigate the expression of HIF-1α and VEGF in HCC and the impacts of the expressions of the two biomarkers on neovascularization and survival in HCC.
Thirty-six patients (32 men, 4 women) with HCC who underwent hepatic resection between March 2000 and October 2001 are included in this retrospective study. The hepatitis B surface antigens are positive in all HCC cases. None of the patients received other therapy before the operation. Median patient age, at time of surgery, is 45.9 (19-77) years. Paraneoplastic liver tissue is taken from non-cancerous tissue 1 cm away from the tumor margin. Six samples of normal liver tissue are taken from the liver tissue around the hepatic hemangioma. All the sections are re-evaluated and the diagnosis are confirmed.
Formalin-fixed, paraffin-embedded tissue specimens are obtained. Serial 4 μmol/L sections are prepared, and one is stained with H&E. Sections are stained for HIF-1α, VEGF and CD34 based on streptavidin-biotin-horseradish peroxidase complex formation as mentioned before[16]. In brief, after deparaffinization and rehydration, endogenous peroxidase is blocked with methanol containing 0.3% hydrogen peroxide for 30 min. Slides are treated with target retrieval solution. Primary antibodies include monoclonal anti-HIF-1α antibody (clone H1alpha67; NEOMARKERS), monoclonal anti-VEGF antibody (clone JH121; NEOMARKERS) and monoclonal anti-CD34 antibody (clone QBEnd/10; NEOMARKERS). The peroxidase reaction is developed, using diaminobenzidine and the slides used are washed and mounted in mountant. Nuclei are lightly counterstained with hematoxylin. Negative controls are performed using PBS instead of the MAb. Two investigators (Bao-An Liu and Seng-Lin Chen) independently evaluate the immunohistochemistry. The immunohistochemical results for HIF-1α and VEGF are classified as follows: -, no staining; +, weak staining; ++, strong staining. Microvessel density, assessed by immunostaining for CD34, is determined according to Weidner[17]: the immunostained sections are scanned at low magnification (×40), and the tumor area with the highest density of distinctly highlighted microvessels (‘hot spot’) is selected. Microvessel density then is determined in the hot spot by counting all vessels at a total magnification of ×200. Each stained lumen is regarded as a single CD34 positive cell is visible, this cell is also interpreted as representing a microvessel.
Survival are calculated from the date of operation. Follow-up is performed through letter and telephone. Median duration of follow-up is 300 d (60-960 d). Follow-up rate is 91.6% (33/36).
Mann-Whitney test and Spearman coefficient of correlation are used as appropriate. Survival curves are calculated using Kaplan-Meier estimates and differences among groups are tested by log rank test. For all tests, a P value of less than or equal to 0.05 is considered as significant. All statistics are calculated through spss 10.0 software.
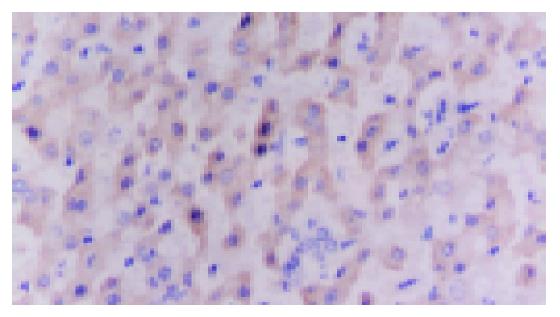
There are no positive staining in negative control. The positive staining is located in the cytoplasma and/or the nucleus (Figure 1). There are 24 sections with positive staining of HIF-1α, including 3 strongly positive sections and 21 weakly positive, among the 36 sections of HCC. However, among the 36 sections of paraneoplastic tissue, all but 2 sections with weakly positive stain of HIF-1α are negative and all the 6 sections of normal liver tissue are negative. The expressions of HIF-1α in the bile duct and the vessels are negative. The expression of HIF-1α in the HCC tissue is statistically significantly higher than that in the paraneoplastic tissue and normal liver tissue (P<0.01).
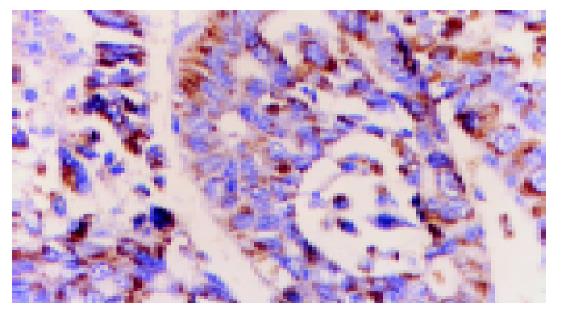
The positive staining of VEGF is located in the cytoplasma (Figure 2). The positive rate of VEGF in HCC is 32/36, including 16 strongly positive and 16 weakly positive. Among 36 sections of paraneoplastic tissue, all but 6 sections with weakly positive staining of VEGF are negative and all the 6 sections of normal liver tissue are negative. The expression of VEGF in the HCC tissue is statistically significantly higher than that in the paraneoplastic tissue and normal liver tissue (P<0.05).
Significant association is only found between HIF-1α and VEGF expression and microscopic venous invasion (P<0.05), among the 5 clinicopathological data including serum alpha fetoprotein concentration, cirrhosis, Edmondson grading, tumor size and microscopic venous invasion (Table 1). The expression of HIF-1α or VEGF in HCC with microscopic venous invasion are significantly higher than those in HCC without microscopic venous invasion (P<0.05).
| n | HIF-1a | VEGF | P | |||
| - | + | - | + | |||
| AFP concentration (ng/mL) | ||||||
| ≤50 | 14 | 2 | 12 | 2 | 12 | |
| >50 | 22 | 10 | 12 | 2 | 20 | >0.05 |
| Cirrhosis | ||||||
| With | 25 | 10 | 15 | 4 | 21 | |
| Without | 11 | 2 | 9 | 0 | 11 | >0.05 |
| Edmondson grading | ||||||
| I-II | 12 | 2 | 10 | 1 | 11 | |
| III-IV | 24 | 10 | 14 | 3 | 21 | >0.05 |
| Tumor size (mm) | ||||||
| ≤50 | 9 | 3 | 6 | 1 | 8 | |
| >50 | 27 | 9 | 18 | 3 | 24 | >0.05 |
| Microscopic venous invasion | ||||||
| With | 24 | 3 | 21 | 1 | 23 | |
| Without | 12 | 9 | 3 | 3 | 9 | <0.05 |
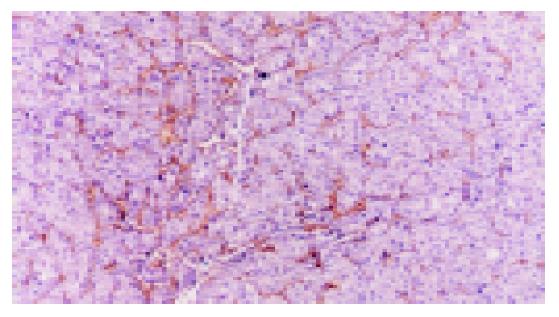
CD34 expression is positive in 33 cases of HCC (Figure 3). Spearman correlation analysis does not only show the expression of HIF-1α as correlated with the expression of VEGF (rs = 0.459, P<0.01), but also shows the expressions of HIF-1α and VEGF as correlated with that of CD34 (rs = 0.412 and 0.336, respectively, P<0.05).
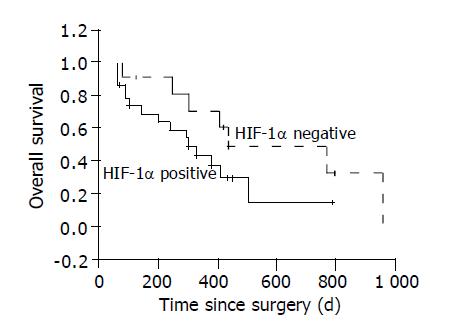
The overall 1, 2-year survival rates of the 36 patients are 52.5% and 28.9%. The 1, 2-year survival rates are 70.7% and 48.0% in HIF-1α negative HCC group, 43.4% and 14.9% in HIF-1α positive group. Log rank test shows the differences among the two groups are not significant (P = 0.10, Figure 4).
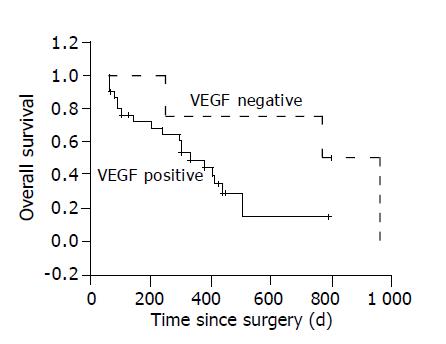
The 1, 2-year survival rates are both 75.0% in VEGF negative HCC group, 49.2% and 14.5% in VEGF positive group. Log rank test shows the differences among the three group are significant (P<0.05, Figure 5).
The rate of respectability of HCC has risen and the 5-year survival rate has improved[1-3]. However, the postoperative relapse rate of HCC remains higher, which becomes one of the main obstacles to improve the survival rate. The molecular mechanisms of the relapse and metastasis of HCC are thus of interest. Neovascularization is one of the key steps[4-6]. HIF-1α is thought to be involved in the regulation of neovascularization in malignancies. The purpose of this study is to determine whether the expression of HIF-1α increases in HCC tissue and the impacts of HIF-1α expression on neovascularization and survival.
Most studies about the effects of HIF-1α on the neovascularization in malignancies have used in vitro or animal models. It has been confirmed that in many cancer cell lines, hypoxia can induce the expression of some proangiogenic factors, including VEGF, insulin-like growth factor, platelet-derived endothelial growth factor, through HIF-1α-dependent manner. It has also been proven through the method of gene knockout that the loss of HIF-1α may significantly suppress the growth of tumors including glioblastoma and malignant teratoma and most importantly decrease the neovascularization of those malignancies[18,19].
HIF-1α is also proven to highly express in many kinds of malignancies, such as stomach, colon, breast, thyroid, pancreas, ovary, lung and otopharyngeal cancer[11,20-23]. Zhong et al[11] and Talks et al[20] have studied the expression of HIF-1α in 8 cases and 5 cases of HCC tissue using immunohistochemistry. The results show that 2 cases out of 13 have positive HIF-1α expression. However, it is evident that the amount of the samples in the two studies is too small to conclude. In the current study, the expression of HIF-1α in 36 cases of HCC are studied through immunohistochemistry and the positive rate is 24/36, higher than that in paraneoplastic liver tissue and normal liver tissue. Also, we demonstrate for the first time to our knowledge that the expression of HIF-1α in HCC does not only have relationship with that of VEGF, but also with that of MVD and microvenous invasion. Thus, this clinical study has supported that HIF-1α may play pivotal role in the neovascularization in HCC through regulation of VEGF expression. Our findings are consistent with some previous reports in other cancers, such as ovarian cancer[21], ductal carcinomas in situ[22], non-small cell lung cancer[23] and supratentorial pure oligodendrogliomas[24].
In the present study, we find no significant difference in survival among HIF-1α negative group and positive group. However, overexpression of HIF-1α is demonstrated to be an independent prognostic factor by univariate and multivariate analysis in some malignancies, such as early-stage invasive cervical cancer[12], non-small cell lung cancer[23], oligodendroglioma[24] and oropharyngeal cancer[25]. Thus to definitely clarify possible differences in survival in HCC, larger scale studies will be needed.
We conclude that overexpression of HIF-1α protein is associated with neovascularization in HCC, possibly through regulation of VEGF transcription.
We thank Bao-An Liu and Seng-Lin Chen for evaluating the immunohistochemical results.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 542] [Cited by in F6Publishing: 552] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 316] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 595] [Cited by in F6Publishing: 642] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 4. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6437] [Cited by in F6Publishing: 6263] [Article Influence: 261.0] [Reference Citation Analysis (0)] |
| 5. | Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2741] [Cited by in F6Publishing: 2639] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 6. | El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 240] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680-5684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 608] [Cited by in F6Publishing: 657] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4392] [Cited by in F6Publishing: 4532] [Article Influence: 156.3] [Reference Citation Analysis (0)] |
| 9. | Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915-3918. [PubMed] [Cited in This Article: ] |
| 10. | Melillo G, Taylor LS, Brooks A, Musso T, Cox GW, Varesio L. Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem. 1997;272:12236-12243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830-5835. [PubMed] [Cited in This Article: ] |
| 12. | Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693-4696. [PubMed] [Cited in This Article: ] |
| 13. | Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1997;94:8104-8109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 790] [Cited by in F6Publishing: 775] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 14. | Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333-13340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 720] [Cited by in F6Publishing: 689] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5' enhancer. Circ Res. 1995;77:638-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 692] [Cited by in F6Publishing: 693] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 16. | Huang GW, Yang LY. Metallothionein expression in hepatocellular carcinoma. World J Gastroenterol. 2002;8:650-653. [PubMed] [Cited in This Article: ] |
| 17. | Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 555] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Jiang BH, Agani F, Passaniti A, Semenza GL. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328-5335. [PubMed] [Cited in This Article: ] |
| 19. | Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005-3015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1171] [Cited by in F6Publishing: 1164] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 20. | Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 928] [Cited by in F6Publishing: 933] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 21. | Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia-inducible factor 1alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res. 2001;7:1661-1668. [PubMed] [Cited in This Article: ] |
| 22. | Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst. 2001;93:309-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 455] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Birner P, Gatterbauer B, Oberhuber G, Schindl M, Rössler K, Prodinger A, Budka H, Hainfellner JA. Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer. 2001;92:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 25. | Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911-2916. [PubMed] [Cited in This Article: ] |