Published online Feb 1, 2004. doi: 10.3748/wjg.v10.i3.455
Revised: July 4, 2003
Accepted: July 30, 2003
Published online: February 1, 2004
AIM: To study the effect of probiotics on interleukin-8 secretion in intestinal epithelia when stimulated by proinflammatory cytokines.
METHODS: Colonic adenocarcinoma HT29 cells were cultured and divided into four groups: control, TNF-α (group T in short), bifidobacterium (group B), lactobacillus (group L). B. Longum and L. bulgaricus were suspended in culture medium with a concentration of 1 × 108 cfu/ml and added into 24 wells respectively. One hour later TNF-α (10 ng/ml) was added into each well of groups T, B, L. The supernatants were collected and measured for IL-8 after 3 hours, nuclear factor-kB (NF-κB) p65 was also examined by Western blotting.
RESULTS: There was less interleukin-8 secretion in HT29 cells when preincubated with B. Longum or L. bulgaricus compared with group T. Less p65 appeared in nuclei in groups B and L compared with group T, as detected by Western blot.
CONCLUSION: Probiotics can suppress interleukin-8 secretion in intestinal epithelia when stimulated by proinflammatory cytokines, which is most likely mediated by NF-κB.
- Citation: Bai AP, Ouyang Q, Zhang W, Wang CH, Li SF. Probiotics inhibit TNF-α-induced interleukin-8 secretion of HT29 cells. World J Gastroenterol 2004; 10(3): 455-457
- URL: https://www.wjgnet.com/1007-9327/full/v10/i3/455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i3.455
Intestinal epithelium is an important factor of gut mucosal barrier, and participates in innate immunity. Intestinal epithelia are capable of releasing some proinflammatory cytokines such as IL-8 when stimulated by cytokines like TNF-α, and can response to enteric pathogens and release some proinflammatory cytokines which in turn direct the movement of inflammatory cells of the lamina propria[1]. Probiotics, including bifidobacterium, lactobacillus play an essential role in the completeness of intestinal mucosa barrier. For example, some probiotic strains could modulate intestinal mucosal immune response, some could play protective roles by inhibiting the adhesion of pathogenic bacteria to intestinal epithelia. The present study was to investigate the effect of probiotics on IL-8 secretion of intestinal epithelium induced by TNF-α, and its possible mechanism.
rh TNF-α was obtained from Pepro Tech Ec Ltd,UK. Rabbit anti-human NF-κB p65 polyclonal antibody, peroxidase-conjugated goat anti-rabbit IgG were purchased from Santa Cruz Biotechnology Inc.,USA. Human IL-8 ELISA kit was supplied by Jingmei Biotech Co. Ltd., China. BHI-agar was provided by Oxoid Co.,UK. TMB membrane peroxidase substrate system was provided by KPL Inc., USA.
Bacteria Bifidobacterium longum and lactobacillus bulgaricus LB10 were provided by the Department of Microbiology, Huaxi School of Stomatology, Si chuan University. The strains were grown at 37 °C in static, nonaerated BHI-agar to reach the mid-log phase. Bacteria were harvested by centrifugation at 2500 g for 15 min at 20 °C. After two washes in sterile PBS pH 7.4, at 25 °C, the bacteria were resuspended in PBS. Cell counts in the bacteria suspension were estimated by optical density at 600 nm absorbance (BioMerieux, Germany). Then the bacteria were added to the cell culture wells at appropriate dilution to reach a final concentration of 108 cfu/ml of medium.
Cells and bacteria coculture HT29 cells were grown in RPMI1640 with 10% fetal calf serum, and divided into four groups: control, TNF-α (group T in short), bifidobacterium (group B), lactobacillus (group L). When grown to confluence in single layer, cells were washed three times with PBS pH 7.4, to remove culture medium and nonadherent cells. The bacteria in culture medium were transferred into individual wells respectively. TNF-α (10 ng/ml) was added into each well of groups T, B, L 1 hour later. The supernatants were collected and centrifuged for measurement of IL-8 after 3 hours.
IL-8 enzyme-linked immunosorbent assays IL-8 enzyme-linked immunosorbent assays (ELISA) were performed according to the manufacturer’s instructions. In short, polyclonal goat anti-human IL-8 antibodies were used as capturing antibodies, biotinylated polyclonal rabbit anti-human IL-8 antibodies as detecting antibodies. Streptavidin-HRP and TMBS were added as color indicator. Plates were read at 450 nm of wavelength right after color reaction was stopped with acid. All procedures were performed at room temperature.
Assessment of NF-κB activation by Western blotting Nuclear extracts were prepared according to the protocol described by Schreiber, et al[2]. Nuclear proteins were separated by SDS-polyacrylamide mini-gel electrophoresis at a constant current of 30 mA for 150 min, then transferred to nitrocellulose membranes and stained with ponceau S to verify equal protein loading. Membranes were blocked in 5% milk in Tris-buffered saline for 4 h at 4 °C, incubated overnight at 4 °C with anti-human p65 polyclonal antibody (at a dilution of 1:1000) followed by 1 h incubation at room temperature with peroxidase-conjugated goat anti-rabbit IgG (1:2000). Finally, the membranes were incubated in TMB membrane peroxidase substrate solution.
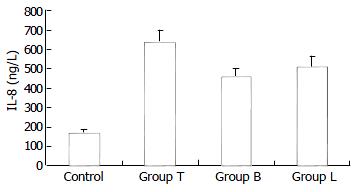
Concentrations of IL-8 in supernatants of each group are shown in Figure 1. The concentration of IL-8 in control was only 172.2 ± 42.1 ng/L. When stimulated by TNF-α, HT29 cells secreted a large number of IL-8, and the concentration of IL-8 in group T was 639.5 ± 62.3 ng/L. However, when preincubated with B. Longum or L. bulgaricus, HT29 cells produced less IL-8, compared with group T, and the concentrations of IL-8 were 461.8 ± 76.7 ng/L, 515.4 ± 55.4 ng/L in groups B and L, respectively.
There was little NF-κB p65 in nuclei of normal epithelia without any stimuli, and it was very difficult to detect p65 in those cells by Western blotting. The epithelia expressed high levels of nuclear NF-κB p65 when stimulated by TNF-α. Decreased expression of nuclear NF-κB p65 appeared in cells of groups B and L, in contrast to group T, as Western blotting showed (Figure 2).
Intestinal epithelia constitute mucosal barrier of the bowel, and participate in inflammatory or immune responses in gut[3,4]. In some gastrointestinal infectious and inflammatory conditions, such as inflammatory bowel disease (IBD), acute gastroenteritis, inflammatory cells including monocytes, lymphocytes, were activated and accumulated in lamina propria. The cells secrete excessive inflammatory products, such as TH1 type cytokines, chemokines and a lot of active oxides. Overproduction of cytokines could affect the biological action of epithelial cells. For instance, TNF-α could induce epithelial cells to secrete IL-8, and express membrane Toll-like receptor 4 (TLR4) excessively[5,6]. TLR4 could enable intestinal epithelia hyperreactive in response to lipopolysaccharides (LPS), the component of bacteria walls, and IL-8 had leukocytes chemotactic and stimulatory properties[7]. As more inflammatory cells infiltrate, the inflammatory reaction is therefore amplified.
The normal flora of human gastrointestinal tract contains diverse populations of bacteria which play an essential role in the development of gut mucosal barrier and innate immunity. Some intestinal microflora could exert a protective role against pathogens[8]. Aberrance of gut microflora has been reported in IBD and acute gastroenteritis[9,10]. The aberrant microflora dysregulates mucosal immune reaction. Invasion of some virulent strains into epithelia could break down the integrity of intestinal mucosa, and induce inflammatory cell infiltration[11]. Some researchers found that manipulating the normal intestinal flora using probiotics had a beneficial effect on health by altering the microbial environment, and some components of the flora could down-regulate inflammation when supplemented to patients with gastrointestinal diseases[12,13]. Some studies have been undergoing to explore the possible mechanisms of probiotic action on gut epithelium and mucosal immune system.
In order to imitate the inflammatory condition of gut in vitro, we used TNF-α to stimulate human colonic adenocarcinoma HT29 cells, which has basically the same biological properties as normal colonic epithelia. As some probiotic strains could adhere to human intestinal cell surface[14,15], two probiotic strains, B.longum and L.bulgaricus, inhibited the secretion of IL-8 in HT29 cells when stimulated with TNF-α one hour after coculture with the two probiotic strains. It indicated that the strains could trigger anti-inflammatory pathways within the gut epithelium. The epithelia attached to the strains showed immune hyporeaction to TNF-α, and produced less IL-8. Because transcriptional control of IL-8 was mediated by transcription factor NF-κB[15], we investigated the expression of nuclear NF-κB p65 in HT29 cells. There was little NF-κB p65 in nuclei of normal epithelia without any stimuli. TNF-α highly up-regulated the expression levels of nuclear NF-κB p65. However, the epithelia pre-cocultured with two probiotic strains before TNF-α treatment showed a decreased expression of nuclear NF-κB p65. We postulated that such normal florae down-regulated inflammation by inhibiting NF-κB activation in gut epithelium.
Neish reported some nonvirulent Salmonella strains attenuated the synthesis of inflammatory effector molecules in model human epithelia elicited by proinflammatory stimuli by blockade of IκB degradation[16]. In order to investigate such an effect of the bacteria in gut, we selected and cultured two strains of bifidobacterium and lactobacillus under anaerobic conditions. The two strains successfully inhibited IL-8 secretion and NF-κB activation in intestinal epithelia in the experiment. At present, some probiotic compounds have been used in management of some diseases, such as maintenance therapy in IBD[17,18]. Although the mechanism of probiotic action has not been fully understood, the beneficial effects were consistent with an anti-inflammatory state conferred by probiotics[19,20]. More researches need to be done in order to understand the beneficial effect of probiotics on human beings.
Edited by Zhu LH and Wang XL
| 1. | Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860-1869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3329] [Cited by in F6Publishing: 3621] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 3. | Gordon JI, Hooper LV, McNevin MS, Wong M, Bry L. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium, and diffuse GALT. Am J Physiol. 1997;273:G565-G570. [PubMed] [Cited in This Article: ] |
| 4. | Campbell N, Yio XY, So LP, Li Y, Mayer L. The intestinal epithelial cell: processing and presentation of antigen to the mucosal immune system. Immunol Rev. 1999;172:315-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 379] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 6. | Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-αlpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2272] [Cited by in F6Publishing: 2206] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 8. | Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation. Gut. 2002;50 Suppl 3:III54-III59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Büschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol. 1995;102:448-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 667] [Cited by in F6Publishing: 622] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 10. | Masseret E, Boudeau J, Colombel JF, Neut C, Desreumaux P, Joly B, Cortot A, Darfeuille-Michaud A. Genetically related Escherichia coli strains associated with Crohn's disease. Gut. 2001;48:320-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Resta-Lenert S, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: role of iNOS and COX-2. Gastroenterology. 2002;122:1070-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Guandalini S. Use of Lactobacillus-GG in paediatric Crohn's disease. Dig Liver Dis. 2002;34 Suppl 2:S63-S65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 802] [Cited by in F6Publishing: 680] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 14. | Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 527] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 15. | Adlerberth I, Ahrne S, Johansson ML, Molin G, Hanson LA, Wold AE. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Environ Microbiol. 1996;62:2244-2251. [PubMed] [Cited in This Article: ] |
| 16. | Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 663] [Cited by in F6Publishing: 690] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 17. | Malin M, Suomalainen H, Saxelin M, Isolauri E. Promotion of IgA immune response in patients with Crohn's disease by oral bacteriotherapy with Lactobacillus GG. Ann Nutr Metab. 1996;40:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 201] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1077] [Cited by in F6Publishing: 913] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 19. | Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 569] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 20. | Shibolet O, Karmeli F, Eliakim R, Swennen E, Brigidi P, Gionchetti P, Campieri M, Morgenstern S, Rachmilewitz D. Variable response to probiotics in two models of experimental colitis in rats. Inflamm Bowel Dis. 2002;8:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |