Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2744
Revised: September 23, 2003
Accepted: October 7, 2003
Published online: September 15, 2004
AIM: To explore the expression of triggering receptor expressed on myeloid cells (TREM-1) mRNA in acute pancreatitis (AP).
METHODS: Using the reverse transcription polymerase chain reaction (RT-PCR), we examined the expression of TREM-1 mRNA in 10 cases of mild acute pancreatitis (MAP), 8 cases of severe acute pancreatitis (SAP), and 10 cases of healthy control subjects. And we also examined the expression of TREM-1 mRNA in 14 cases of AP (including 10 MAP and 4 SAP) before treatment, after successful therapy and clinically cured.
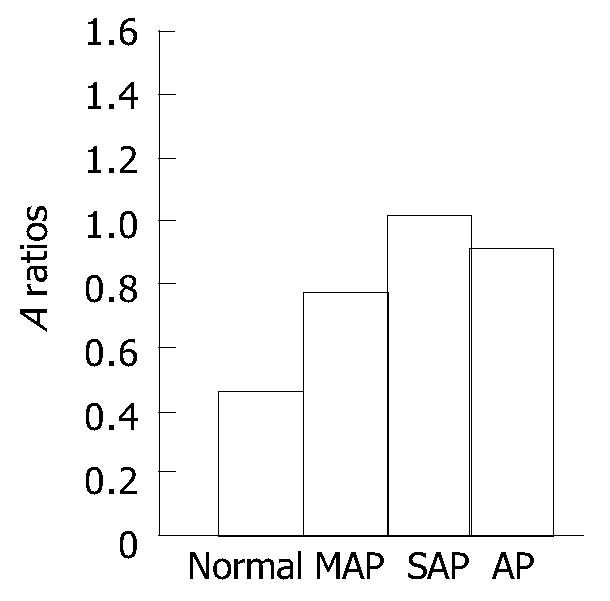
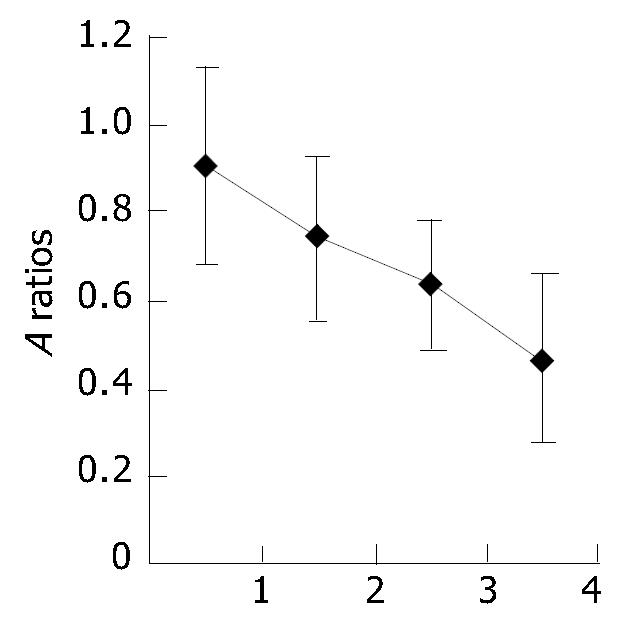
RESULTS: The expression of TREM-1 mRNA in the groups of MAP, SAP patients and healthy control subjects was 0.771 ± 0.274, 1.092 ± 0.331 and 0.459 ± 0.175, respectively; there was a significant difference among the three groups (P < 0.05). And there was also a significant difference between the AP patients (0.914 ± 0.341) and healthy control subjects (0.459 ± 0.175) (P < 0.05). Moreover, in the 14 cases of AP, before treatment, after successful therapy and clinically cured, the expression of TREM-1 mRNA was 0.905 ± 0.226, 0.739 ± 0.169 and 0.633 ± 0.140, respectively, and there was a significant difference among the three stages (P < 0.05).
CONCLUSION: The expression of TREM-1 mRNA in the patients with AP increases obviously, and correlates with the degree of AP. Furthermore, the expression of TREM-1 mRNA is distinctly different at the different stages of AP. It indicates TREM-1 may play an important role in the occurrence and development of AP.
- Citation: Wang DY, Qin RY, Liu ZR, Gupta MK, Chang Q. Expression of TREM-1 mRNA in acute pancreatitis. World J Gastroenterol 2004; 10(18): 2744-2746
- URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2744.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2744
TREM-1 is a new activating receptor of the Ig superfamily expressed on human myeloid cells. And it is selectively expressed on blood neutrophils and a subset of monocytes and up-regulated by bacterial lipopolysaccharide (LPS)[1]. Researchers have demonstrated that TREM-1 mediates activation of neutrophil and monocytes, and may have a predominant role in inflammatory responses such as septic shock and systemic inflammatory response syndrome (SIRS)[1,2]. In this study, we aimed to explore the expression of TREM-1 mRNA in AP and tried to reveal etiology of AP and underlying mechanism.
Data obtained from 18 AP patients without any treatment during 48 h of the onset of the disease and 10 healthy control subjects were explored. According to the standards of diagnosis and classification of AP proposed at the Sixth National Conference on Pancreatic Surgery of China in 1996[3], we divided the AP patients into two groups: 10 cases of MAP, and 8 of SAP. Among the AP patients, we examined the expression of TREM-1 in 14 patients at different stages of the disease (before treatment, after successful therapy and clinically cured). We collected 2 mL of blood from the patients and controls before breakfast in the morning and stored them in the EDTA tube. The total leucocytes in the blood were separated by centrifugation at 1200 r/min for 15 min.
Trizol reagents, Oligo (dt) 15, M-MLV reverse transcriptase and DEPC were the products of Promega Co. (USA). Taq DNA polymerase were purchased from Sangon Co. (Shanghai). The RNase inhibitor was from Takara Co. (Japan). According to the human TREM-1 and β-actin cDNA sequence reported in GeneBank, we designed two sets of primers: TREM-1 sense 5’-GTGTGTGATCTACCAGCCT-3’, antisense 5’-TTCAGAGTCAGGAGTGGAG-3’ (amplified fragment length: 232 bp); β-actin sense 5’-GTGCGTGACATTAAGGAG-3’, antisense 5’-CTAAGTCATAGTCCGCCT-3’ (amplified fragment length: 520 bp).
The total RNA was extracted from the leucocytes by Trizol reagent. The extracted RNA concentrations and purity were determined at A260 and A280 spectrophotometrically (Beckman DU650, USA). Then 750 mL/L ethanol was used to preserve total RNA at -20 °C.
The total RNA was denatured by incubation for 5 min at 70 °C and rapidly cooled on ice for about 2-3 min. Single-stranded cDNA was synthesized by mixing 1-2 µg denatured RNA with 0.5 µL (40 u/µL) RNase inhibitor, 1 µL (200 u/µL) M-MLV reverse transcriptase, 4 µL reaction buffer, 1 µL Oligodt (15), 1 µL (10 mmol/L) dNTP, and adding DEPC-treated water up to 20 µL. The RT reaction mixture was incubated at 37 °C for 60 min, followed by 95 °C for 5 min to inactivate M-MLV reverse transcripatase, and stored at 4 °C for use.A 5 µL of the cDNA was used as template in PCR amplification for TREM-1. The mixture (50 µL) consisting of 1 µL of primers (p1/p2 of TREM-1 and β-actin) each, Taq DNA polymerase (0.5 µL (5 U/µL),10 × PCR buffer 5 µL, MgCL2 3 µL (25 mmol/L), by addition of deionised water up to 50 µL, was reacted on a programmable thermocycler (Perkinelmer 9600). The DNA was predenatured at 95 °C for 5 min, followed by 35 cycles of denaturation (45 s at 95 °C), annealing (45 s at 56 °C), and extension (45 s at 72 °C). Then the mixture was finally extended for 10 min at 72 °C, and stored at -20 °C. β-actin was served as internal control in each experiment under the same conditions.
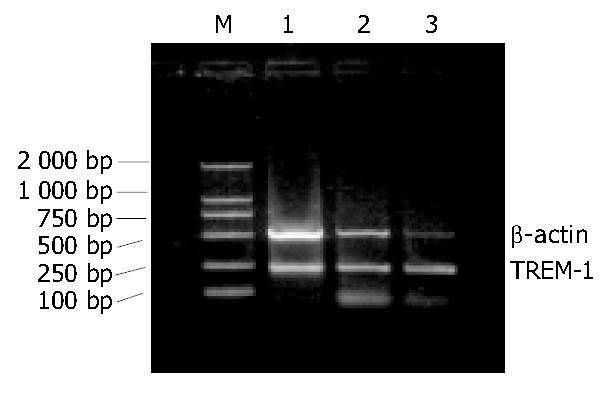
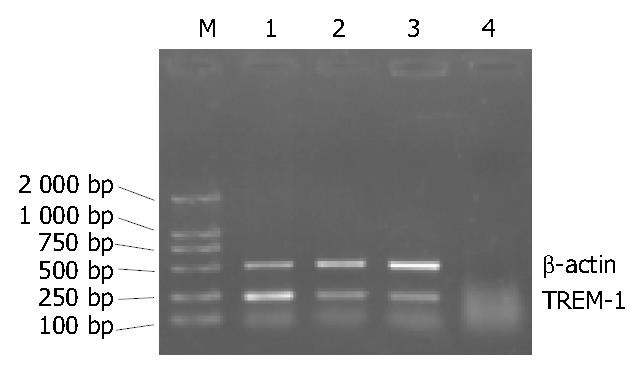
PCR products (7-9 μL) with DL 2000 marker were electrophoresed through 10 g/L agarose gel in 1 × TBE at 70 V for 30 min. The samples were stained with ethidium bromide (0.5 mg/L) and observed by ultraviolet illumination and photographed. Each lane was scanned longitudinally. The A values of TREM-1 and β-actin bands were measured by a gel imaging analysis system (GELWORKS 1D advanced v4.01). The A ratios of TREM-1/β-actin representing the relative levels of the expression of TREM-1 mRNA were calculated for semi-quantitative analysis.
Results were presented as mean ± SD. Statistical comparisons were analyzed through the SAS 8.1 software. Analysis of variance was used. P < 0.05 was considered statistically significant.
The expression of TREM-1 mRNA in the groups of MAP, SAP patients and healthy control subjects were 0.771 ± 0.274, 1.092 ± 0.331 and 0.459 ± 0. 175, respectively. There was a significant difference among the three groups (P < 0.05). The difference was also significant (P < 0.05) (Figure 1, Figure 2) between the AP patients (0.914 ± 0.341) and healthy control subjects (0.459 ± 0.175). In AP patients of before treatment, after successful therapy and clinically cured, the expression of TREM-1 mRNA was 0.905 ± 0.226, 0.739 ± 0.169 and 0.633 ± 0.140, respectively.
There was a significant difference among the three stages (P < 0.01 or P < 0.05). And the expression of TREM-1 mRNA was higher in clinically cured group than in healthy control subjects (P < 0.05) (Figure 3, Figure 4).
AP is a disease of self-digestive chemical inflammation with unclear etiology. In most cases, they present a self-limited course. Twenty percent of patients with AP have a severe form of the disease. SAP is such a severe disease that it may be associated with both systemic and local complications, such as respiratory insufficiency with adult respiratory distress syndrome (ARDS), renal failure, the development of pancreatic and peripancreatic infection. The mortality of SAP is higher than 40%[4,5]. Even now, there are no efficient and sensitive methods to treat AP successfully because the pathogenesis of AP still remains obscure[6]. Recent studies[7] show that factors like the pancreatic enzymes activated abnormally lead the body to secrete and amplify large quantities of cytokines. Finally, the cytokines increase vascular permeability, activate complement components, resulting in microcirculatory impairment and imbalance of thrombo-fibrinolytic system. Many of these events occur not only in the pancreas itself, but also in the other vital organs and tissues, leading to SAP and complications[8,9]. Generally, there are three phases of AP progression: local acinar injury, systemic response, and general sepsis[7]. Moreover, some data suggest that the changes in concentration of proinflammatory cytokines can be an early diagnostic criterion for AP[10,11]. Most authors have realized that the pathogenesis of SAP is very complicated. It is a multifactorial as well as multifaceted disease. First of all, the etiologic agents initiate the pancreatic acinar injury by release of pancreatic enzymes and over-stimulation of macrophages and neutrophils, and then the cytokines and inflammatory mediators are delivered[12,13].
In 2000, Bouchon et al[14] had identified a new activating receptor of the Ig superfamily expressed on human myeloid cells, called triggering receptor expressed on myeloid cells (TREM). TREM-1 is selectively expressed on blood neutrophils and a subset of monocytes and up-regulated by bacterial LPS. Engagement of TREM-1 triggers secretion of IL-8, monocyte chemotactic protein-1, and TNF-alpha and induces neutrophil degranulation. Intracellularly, TREM-1 induces Ca2+ mobilization and tyrosine phosphorylation of extracellular signal-related kinase 1 (ERK1), ERK2 and phospholipase C-gamma. To mediate activation, TREM-1 associates with the transmembrane adapter molecule DAP12. Furthermore Bleharski et al[15] suggested that activation of TREM-1 on monocytes participated in the early induced and adaptive immune responses involved in host defense against microbial challenges. Thus, TREM-1 was considered mediating the activation of neutrophil and monocytes, and might have a predominant role in inflammatory responses.
Wilson et al[16] discovered that SAP had many similarities to sepsis syndrome and septic shock. The haemodynamic features of cardiovascular instability, reduced ejection fraction and decreased systemic vascular resistance were indistinguishable in each of these conditions. In addition, there are many striking similarities in the cytokine and inflammatory mediator profiles, suggesting that the haemodynamic abnormalities may result from the same pathogenic mechanisms, albeit as a result of different inflammatory stimuli.
In 2001 through their experiments, Bouchon et al[2] believed that inflammatory responses to microbial products were amplified by a pathway mediated by TREM-1. TREM-1 was an activating receptor expressed at high levels in neutrophils and monocytes that infiltrate human tissues infected with bacteria. Furthermore, it was up-regulated in peritoneal neutrophils of patients with microbial sepsis and mice with experimental LPS-induced shock. Notably, blockade of TREM-1 protected mice against LPS-induced shock, as well as microbial sepsis caused by live Escherichia coli or caecal ligation and puncture. Their results demonstrate a critical function of TREM-1 in acute inflammatory responses to bacteria and implicate TREM-1 as a potential therapeutic target for septic shock. A recent study shows that TREM-1 amplifies Toll-like receptor-initiated responses against microbial challenges and potentiates the secretion of proinflammatory chemokines and cytokines in response to bacterial and fungal infections. Blockade of TREM-1 reduced inflammation and increased survival in animal models of bacterial infections that caused systemic hyper-inflammatory syndromes[17]. But nobody knows how TREM-1 expressed in AP as yet. According to the theory mentioned above, we carried out a study of the expression of TREM-1 in AP.
We demonstrated that the expression of TREM-1 in leucocytes of patients with AP was much higher than that in healthy control subjects and correlated with the degree of AP. Furthermore, significant differences in the expression of TREM-1 mRNA were found at the different stages of AP. The expression of TREM-1 decreased gradually with the improvement of severity of the AP patients. This experiment indicates that the expression of TREM-1 is relevant to progression of AP. Because of lack of relevant data about the patients after they were discharged from the hospital, we only compared the expression of TREM-1 between the cured group and healthy control subjects and found that there was still a significant difference between them. Therefore, we suggest that the pathological changes of the pancreas continued even after the patients were cured clinically.
This study may help reveal the etiology of AP and the mechanism of its progression. It may provide a new standard for the diagnosis and classification of AP as well as an efficient method to cure AP by blocking the expression of TREM-1.
Edited by Zhang JZ and Zhu LH Proofread by Xu FM
| 1. | Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991-4995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 807] [Cited by in F6Publishing: 854] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 2. | Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103-1107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 757] [Cited by in F6Publishing: 775] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 3. | The Pancrea Study Group, Chinese medical association of sugery. The standards on diagnosis and classification of acute pancreatitis (1996). Zhonghua Waike Zazhi. 1997;35:773-775. [Cited in This Article: ] |
| 4. | Abu-Zidan FM, Bonham MJ, Windsor JA. Severity of acute pancreatitis: a multivariate analysis of oxidative stress markers and modified Glasgow criteria. Br J Surg. 2000;87:1019-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1929] [Cited by in F6Publishing: 1716] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 6. | Nam JH, Murthy S. Acute pancreatitis--the current status in management. Expert Opin Pharmacother. 2003;4:235-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Bentrem DJ, Joehl RJ. Pancreas: healing response in critical illness. Crit Care Med. 2003;31:S582-S589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Zhang Q, Ni Q, Cai D, Zhang Y, Zhang N, Hou L. Mechanisms of multiple organ damages in acute necrotizing pancreatitis. Chin Med J (Engl). 2001;114:738-742. [PubMed] [Cited in This Article: ] |
| 10. | Riché FC, Cholley BP, Laisné MJ, Vicaut E, Panis YH, Lajeunie EJ, Boudiaf M, Valleur PD. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery. 2003;133:257-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Demydov VM, Zaporozhchenko BS, Demidov SM. [Changes of cytokine levels in the serum in patients with acute pancreatitis as an early symptom for diagnosis]. Klin Khir. 2003;3:29-32. [PubMed] [Cited in This Article: ] |
| 12. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 501] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 25] [Reference Citation Analysis (0)] |
| 14. | Nochi H, Aoki N, Oikawa K, Yanai M, Takiyama Y, Atsuta Y, Kobayashi H, Sato K, Tateno M, Matsuno T. Modulation of hepatic granulomatous responses by transgene expression of DAP12 or TREM-1-Ig molecules. Am J Pathol. 2003;162:1191-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, Modlin RL. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170:3812-3818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Wilson PG, Manji M, Neoptolemos JP. Acute pancreatitis as a model of sepsis. J Antimicrob Chemother. 1998;41 Suppl A:51-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187 Suppl 2:S397-S401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |